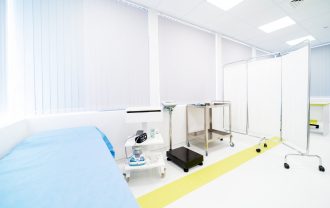
Comac Medical’s Clinical Research Unit (CRU) for Phase I, BA/BE studies has 16 years of experience in early clinical research including over 257 completed clinical trials. The Unit’s facility is the largest one in Southeastern Europe and comprises 42 available beds, on-site Pharmacy. Our specialized teams are dedicated to the conduct of early-phase clinical pharmacology studies in healthy volunteers, special populations, and patient populations over a range of diseases.
Comac Medical’s Phase I Clinical Research Unit is fully integrated with the services Comac Medical offers as a CRO, presenting a clear advantage to our clients.
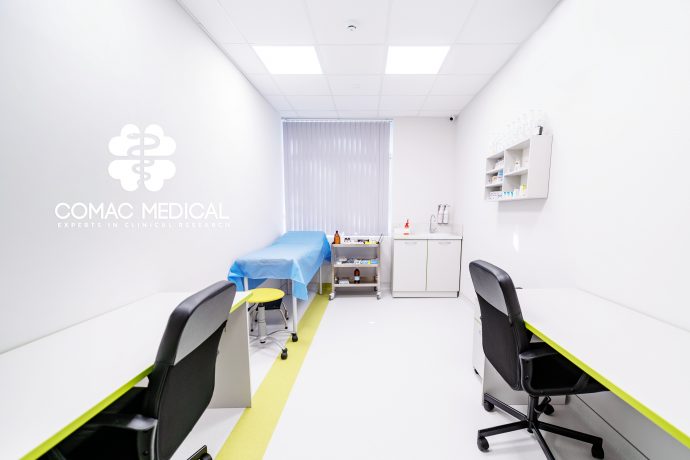
- Area of 1200 m2 (access control system)
- 42 hospital beds (including 1 intensive care bed)
- Dedicated medical team (physician present 24H)
- 24H Emergency Care team
- Possibility to separate cohorts/ trials
- Sampling room
- Pharmacy
- Archiving facility
- Emergency Care equipment (cardiac monitoring)
- 24H Ambulance
- Temperature monitoring & recording system
- Laminar Flow Cabinet for aseptic drug preparation
- Central clock system
- Patient alarm system
- Hi-tech camera monitoring system
- Announcement and audio system
- Monochromatic light rooms
- Power back-up
- Fire-alarm system