
Clinical trials are the backbone of medical advancements, they ensure that new treatments undergo rigorous evaluation for safety and efficacy before reaching patients. However, without stringent oversight, clinical research poses risks, including ethical violations, data privacy breaches, and potential harm to trial participants. To safeguard trial integrity and participant well-being, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) introduced the Good Clinical Practice (GCP) guidelines in 1996, establishing a globally accepted ethical and scientific framework for conducting clinical trials.
Since the ICH GCP E6(R2) amendment in 2016, clinical research has undergone rapid transformation, driven by advances in technology, decentralised trial models, and increasing regulatory expectations. The latest ICH GCP E6(R3) guidelines represent a monumental shift, modernising GCP standards to align with the evolving landscape of clinical research.
Now just past the adoption stage, ICH GCP E6(R3) changes are set for implementation, with the USA and Europe leading the way in integrating these updated guidelines. For Clinical Research Organisations (CROs), this marks a pivotal moment, requiring strategic adaptation to ensure compliance and operational efficiency.
In this article, we will examine the key E6(R3) changes being introduced, explore their impact on CROs, and discuss the challenges and opportunities that lie ahead in this new era of clinical research.
1. Evolution of ICH GCP Guidelines: From Inception to E6(R3)
The Genesis of ICH GCP: Why the Guidelines Were Created
The origins of Good Clinical Practice (GCP) can be traced back to ethical concerns in medical research, particularly following the Nuremberg Trials (1947), which exposed unethical human experimentation during World War II. This led to the Nuremberg Code, emphasising the need for a scientific basis in research on human subjects and voluntary consent in research. Building upon these principles, the Declaration of Helsinki (1964) established ethical guidelines for medical research involving human subjects, reinforcing the need for scientific validity and patient protection.
Regulatory discrepancies developed as clinical research expanded worldwide, calling for a unified framework, therefore, in 1996, the International Council for Harmonisation (ICH) introduced the E6 GCP guidelines, setting a unified ethical and scientific standard for designing, conducting, and reporting clinical trials.
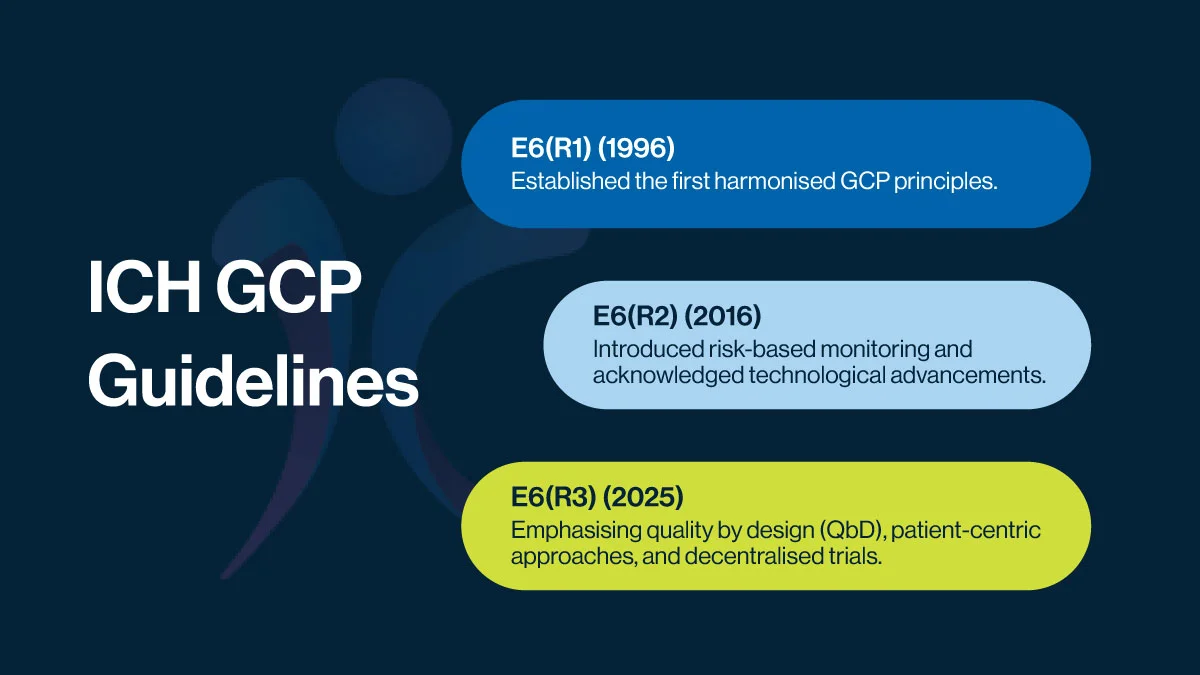
Key Milestones Leading to latest ICH GCP Guidelines
Since its creation, ICH GCP has undergone revisions to adapt to evolving research landscapes, the table below summarises the key amendments to the guidelines.
The latest ICH GCP E6(R3) (2025) update reflects a paradigm shift in clinical research, aligning with modern trial methodologies, digital advancements, and an increased focus on patient needs.
2. Understanding the ICH GCP E6 (R3) Guidelines: What’s New?
These updates aim to enhance trial efficiency while maintaining scientific integrity and ethical responsibility. Here’s a closer look at the key changes:

Structure and Principles
The first noticeable difference is the structure, as illustrated in the above figure:
It begins with an Introduction, establishing the purpose, scope, and overall structure of the guideline. This is followed by a detailed section on Principles, which lays out the foundational ethical standards and core concepts designed to protect the rights, safety, and well-being of trial participants.
Annex 1 defines the roles and responsibilities of key parties, IRBs/IECs, Investigators, and Sponsors—and emphasizes the importance of data governance. Annex 2 builds on this by introducing Risk-Based Quality Management (RBQM) and the integration of Quality by Design (QbD) principles, encouraging proactive risk identification and mitigation from the start of a trial.
A Glossary section ensures clarity by defining essential terms, while the Appendices provide supporting documents such as the Investigator’s Brochure, protocols, and essential records needed for trial conduct and oversight.
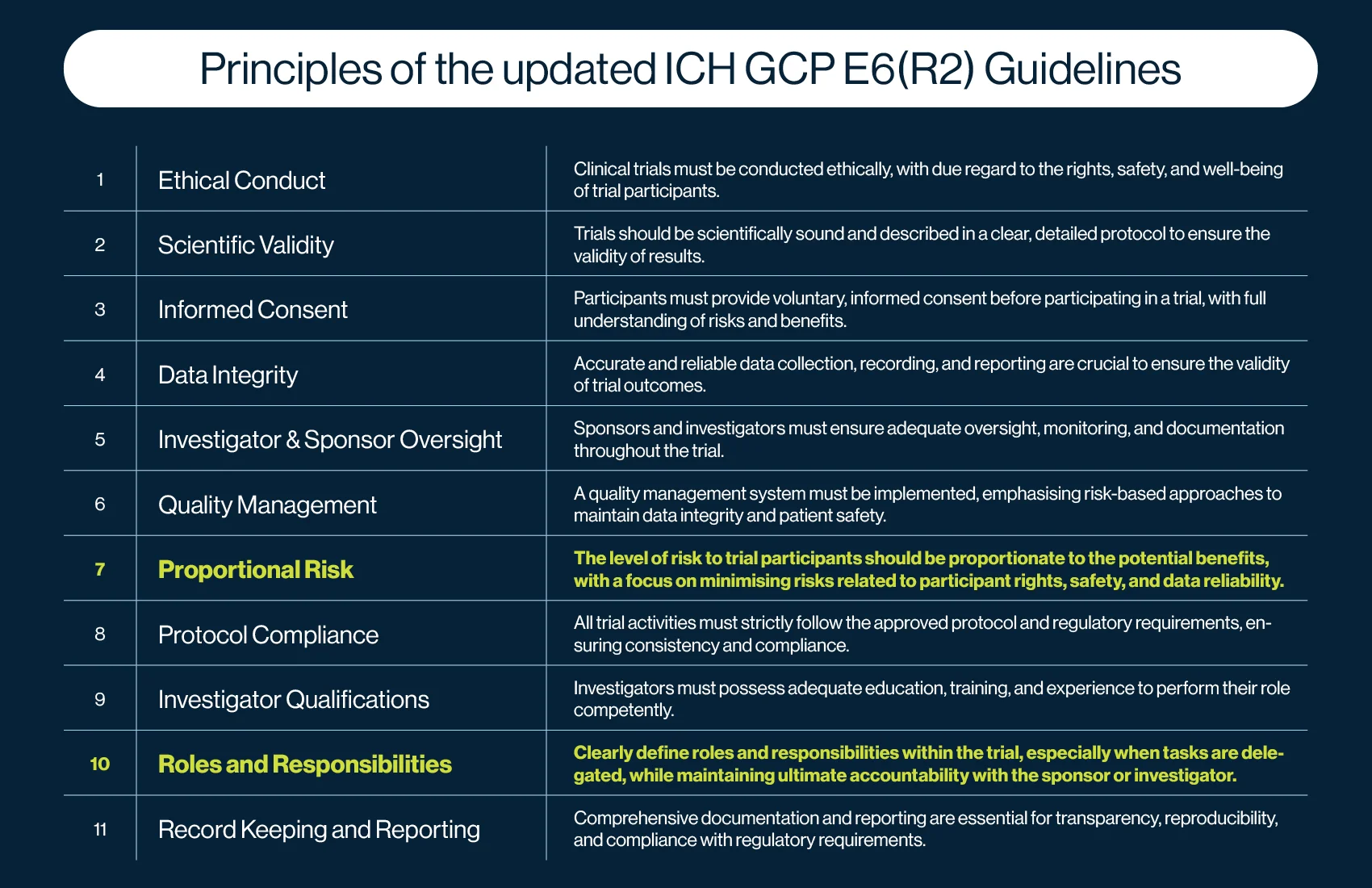
New Principles
The ICH GCP E6(R3) guidelines introduce 11 ICH GCP principles, compared to 13 in E6(R2). While the core intent of the principles in E6(R2) and E6(R3) remains similar, their application and focus have evolved, with the addition of two new principles: Principle 7 (Proportional Risk) and Principle 10 (Roles and Responsibilities). Several existing principles have also been consolidated or refined to enhance clarity and efficiency.
Principle 7 emphasises that the risks to participants’ rights, safety, and well-being, as well as the reliability of trial results, should be proportionate to the benefits of the research. While risk mitigation was already embedded in E6(R2), this new principle places greater focus on ensuring that risk is not an undue burden on trial participants.
Principle 10 reinforces the need for clear delegation of roles and responsibilities in clinical trials. It requires sponsors and investigators to explicitly define delegated tasks while maintaining overall accountability for trial oversight, which is a responsibility that has always been central to GCP.
These refinements reflect a stronger emphasis on patient protection, risk-based thinking, and structured oversight, aligning with modern clinical research practices.
Quality by Design (QbD) and Risk-Based Approaches
The E6(R3) discusses the Quality by Design (QbD) approach, which prioritises proactive risk management in clinical trials rather than reactive problem-solving. This means that instead of focusing solely on compliance and relying on quality assurance audits to correct issues after they occur, the quality by design approach incorporates quality principles directly into trial design from the beginning.
The ICH GCP E6(R3) aligns closely with ICH E8(R1), which emphasises a risk-based and quality-focused approach throughout the clinical trial lifecycle. This connection ensures that quality by design and risk management principles are consistently applied from study planning to execution.
The updated guidelines aim to identify and mitigate foreseeable risks to data integrity and patient safety from the outset, ensuring that trial integrity remains robust. Additionally, the risk-based quality management principles of QbD enable sponsors and CROs to address critical risks early, reducing protocol deviations and inefficiencies while safeguarding trial integrity.
Emphasis on Patient-Centricity
E6(R3) also highlights the importance of patient involvement in trial design, in particular, recognising that participant engagement leads to better recruitment, retention, and data quality. This shift is in line with global regulatory trends, including the FDA’s Patient-Focused Drug Development (PFDD) initiative. The guidelines encourage decentralised clinical trials (DCTs), remote monitoring, and flexible study designs which reduce the burden on patients while maintaining data robustness. There is also an increased focus on transparency of clinical trials results with participants and by means of respecting their preferences.
Modernised Data Management and Technological Integration
With improved technological innovations, such as usage of electronic health records (EHRs), the new ICH GCP guidelines for clinical data management have provided further clarification about on digital data integrity, cybersecurity, and remote trial conduct. The update continually mentions remote as well as real-time data monitoring which ensures compliance with evolving GDPR and FDA 21 CFR regulations on data security. Additionally, the QbD approach is also applicable to the quality of electronic data used wherein investigators’ accountability for monitoring data integrity and the quality of the electronic systems they use have been increasingly underscored.
3. Implications for Service Providers
The adoption and implementation of the new guidelines of ICH GCP E6(R3) presents both challenges and opportunities for service providers such as CROs, who must align their operations, training, and technological capabilities with the updated guidelines to maintain compliance and ensure high-quality clinical research.
Operational Adaptations
To meet the new E6(R3) standards, service providers must revise their standard operating procedures (SOPs) and integrate the more rigorous risk-based quality management into operations, which may require additional staff training by means of interdisciplinary programs to emphasise the Quality by Design, patient-centric approaches, and modernised data management requirements. Additionally, E6(R3) states that service providers must focus on protocol feasibility and “avoid unnecessary complexity” to minimise future changes to trials, while ensuring trials are both scientifically sound and operationally efficient.
In terms of timelines, regulatory agencies such as the FDA and EMA are expected to enforce E6(R3) within the course of the following months, with the USA and Europe leading early adoption. CROs should anticipate a phased implementation period, during which sponsors and regulators will expect demonstrable progress in aligning with the new standards.
Enhancing Collaboration with Sponsors and Investigators
The increased focus on risk-based trial design and execution necessitates stronger communication and collaboration among CROs, sponsors, and investigators. ICH GCP E6(R3) also states a new principle wherein clear definition of roles and responsibilities under risk-based quality management frameworks will ensure risk assessment is proactive rather than reactive. Enhanced data-sharing strategies, risk assessment meetings, and transparent reporting structures will be critical to maintaining regulatory compliance and trial efficiency.
Leveraging Technology for Compliance
To meet E6(R3) requirements, CROs must embrace digital transformation by implementing advanced electronic data capture (EDC) systems, remote monitoring tools, and AI-driven analytics. The adoption of cloud-based clinical trial management systems (CTMS) and electronic source data (eSource) will streamline compliance with modern data integrity expectations. Additionally, automation in risk detection will allow CROs to identify protocol deviations in real time, reducing costly trial disruptions.
By modernising workflows and enhancing collaboration, CROs can turn regulatory adaptation into a competitive advantage, ensuring they remain trusted partners in the evolving clinical research landscape.
4. Challenges and Considerations in Implementing E6 (R3)
Successful implementation of E6(R3) requires strategic planning, collaboration, and investment in both technology and workforce development.
A major challenge is the adaptation of existing processes to follow the new guidelines, requiring updates to relevant documentation, extensive staff training, and revised risk-based quality management strategies. Organisations would need to eventually ensure seamless integration of Quality by Design principles into trial planning, which demands a shift in mindset from compliance-focused monitoring to proactive risk management. Additionally, the adoption of new technologies would urge investment in infrastructure and cybersecurity to meet evolving regulatory expectations.
CROs and sponsors must stay ahead of evolving regulatory interpretations and ensure alignment with local regulatory authorities to maintain operational efficiency and compliance too.
The ICH GCP E6(R3) guidelines mark a significant evolution in clinical research, highlighting Quality by Design, risk-based approaches, patient-centricity, and modernised data management. While implementation presents challenges, proactive adaptation, technological integration, and strong collaboration will enable CROs and sponsors to enhance trial efficiency, compliance, and participant safety in the evolving regulatory landscape.
To read the full guidelines and learn more about the ICH GCP E6(R3), please click here.



