Introduction
Central and Eastern Europe (CEE) has become a leading destination for clinical trials, offering a compelling mix of benefits for global sponsors. These include cost-efficient operations, a streamlined regulatory environment, and access to diverse, treatment-naïve patient populations. In our previous article, “Benefits of Conducting Clinical Trials in Central and Eastern Europe (CEE),” we explored how the region’s evolving healthcare infrastructure and expertise make it an increasingly attractive option for clinical research.
One of the region’s most significant advantages is its large and diverse patient pool. Countries in CEE are home to treatment-naïve populations with limited prior exposure to medical therapies, ensuring clean and reliable data for clinical trials. Coupled with high patient trust in clinical research and strong recruitment rates, this allows for faster enrollment and reduced study timelines, providing a strategic edge to sponsors.
This article focuses on the top-performing countries in the region: Poland, Hungary, Bulgaria, Romania, and the Czech Republic—each of which combines a robust healthcare system, skilled professionals, and alignment with European Union (EU) regulatory standards. By examining these nations’ unique strengths and shared advantages, this article provides a comprehensive guideline for sponsors looking to navigate the CEE clinical trial landscape effectively.
1. Clinical Trial Landscape in Poland: A Regional Powerhouse
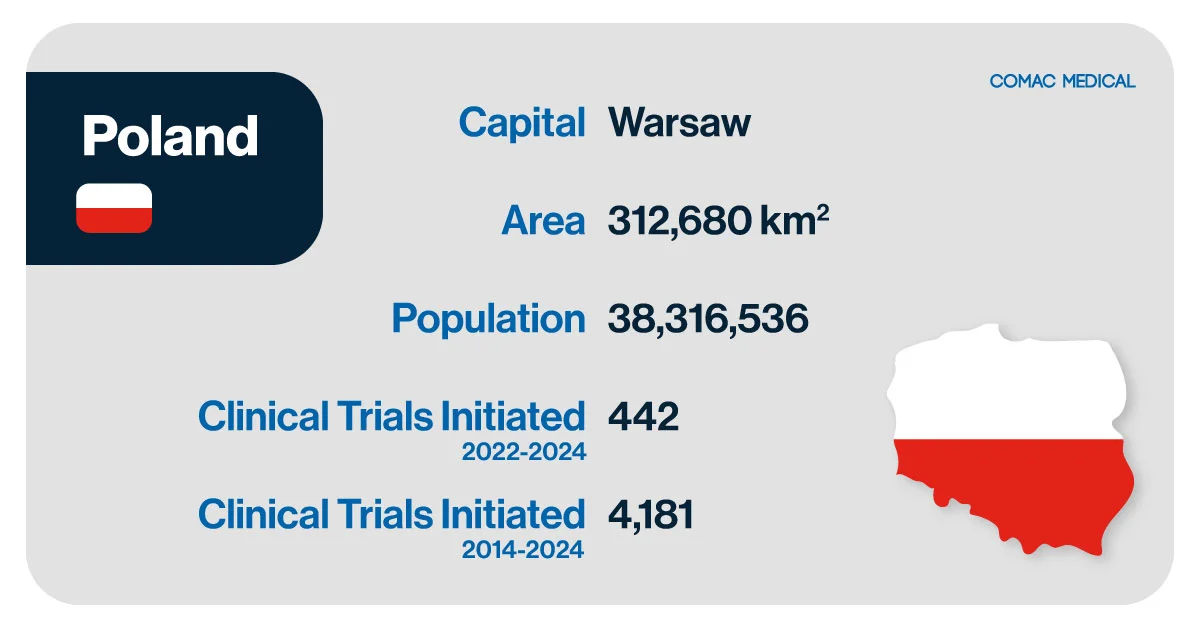
Poland, the ninth-largest country in Europe, plays a pivotal role in the clinical trial landscape within CEE. With a population exceeding 38 million and a robust healthcare infrastructure, the country presents an attractive destination for clinical trial sponsors seeking efficient patient recruitment, regulatory compliance, and high-quality data. Poland’s adherence to EU Clinical Trials Regulations further enhances its appeal as a clinical trial hub. The country has a significant proportion of treatment-naïve patients and high recruitment rates, often outperforming many Western European countries.
Regulatory Environment & Approval Timelines
As an EU member state, Poland follows the European Union Clinical Trials Regulation (EU CTR), specifically Regulation (EU) No 536/2014. This regulation aims to streamline and harmonize the clinical trial approval process across Europe, ensuring a standardized approach to ethical review and regulatory submissions.
Poland’s national regulatory body, the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products (URPL), oversees clinical trial approvals and ensures compliance with both national and EU regulations. The ethics committee processes are well-established, contributing to the country’s reputation for regulatory efficiency. A clear and efficient regulatory framework reduces the time required to initiate a clinical trial, helping sponsors achieve faster study start-ups while maintaining high standards of patient safety.
While the actual timeline may vary depending on the complexity of the trial, Poland’s adherence to the EU CTR helps streamline the process, ensuring that clinical trial applications are approved within 60 to 90 days. Faster approval timelines translate to quicker trial initiation and lower costs for sponsors. This efficiency is critical in competitive markets where time-to-market can make a significant difference in a product’s success.
Key Therapeutic Areas
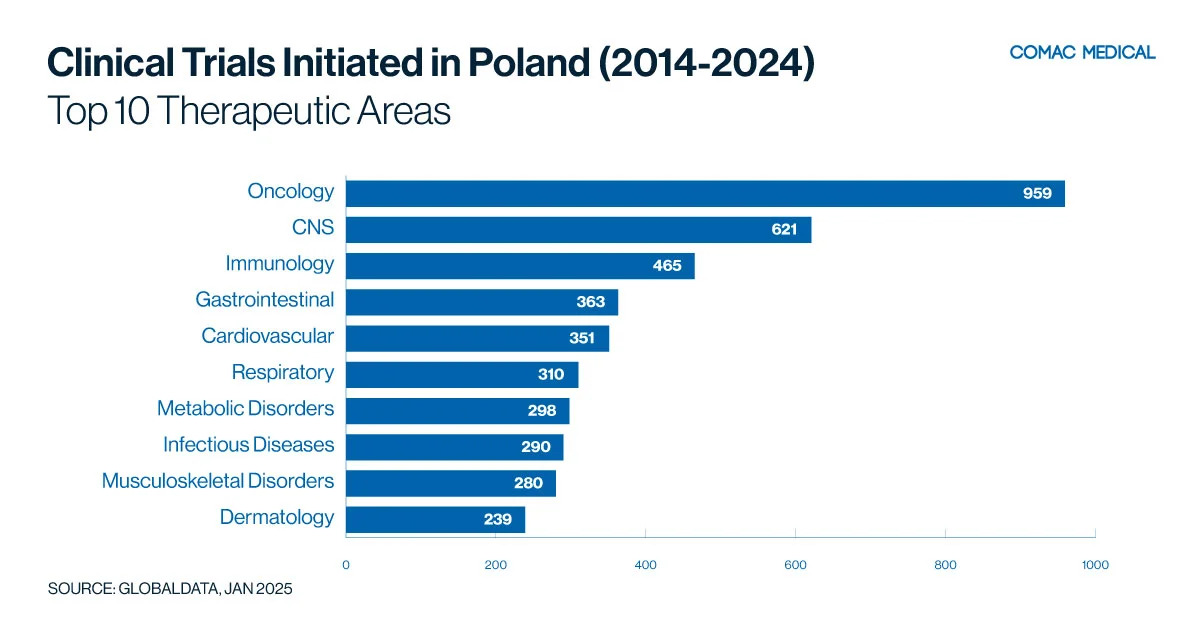
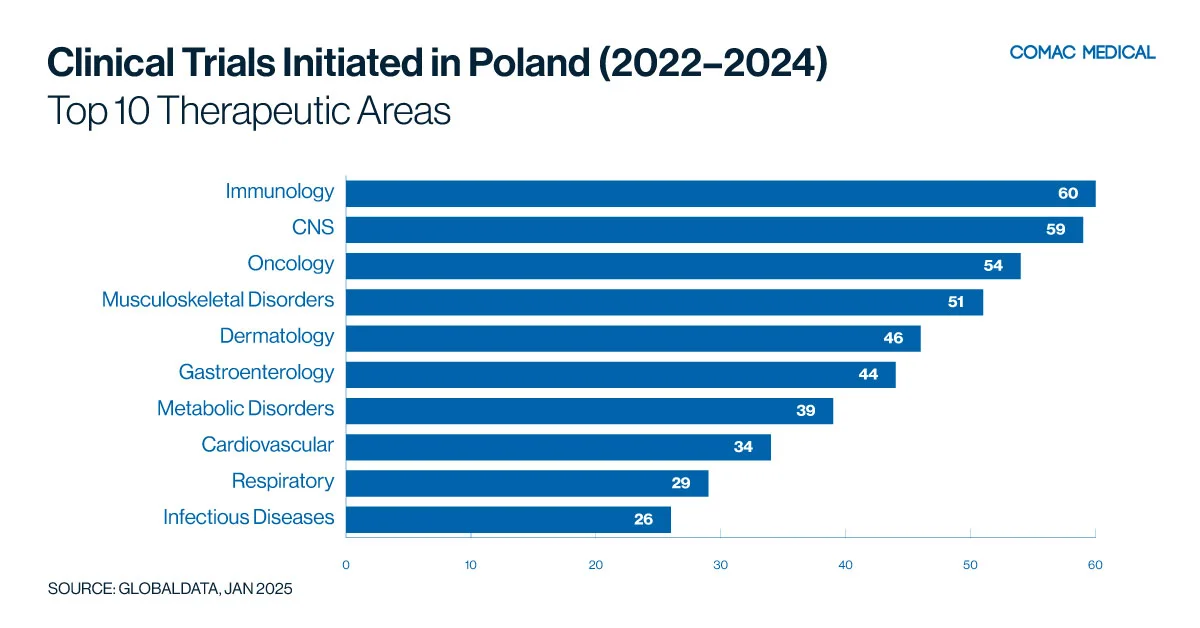
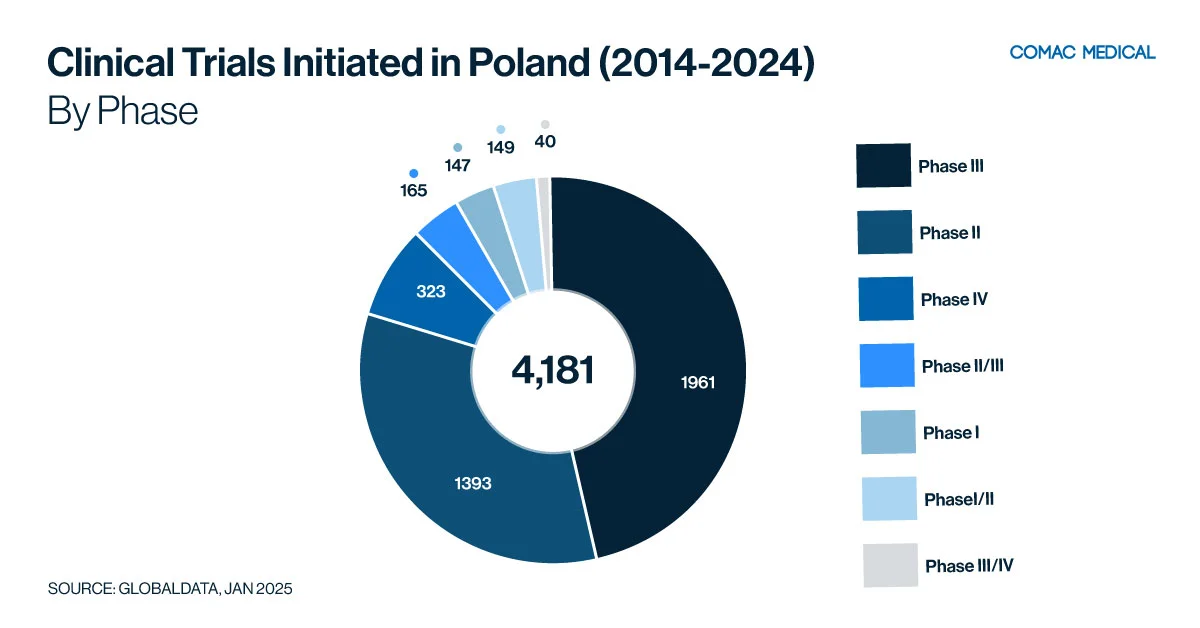
Poland’s clinical trial landscape is particularly active in immunology, neurology, and oncology. These trends have remained consistent from the last 10 years (Figure 1(A), Figure 1(B)) These therapeutic areas are aligned with the country’s health priorities, given the high prevalence of immunological and neurological conditions and the apparent increasing incidence of cancer.
Poland’s specialized cancer centers and experienced investigators in oncology trials provide a strong foundation for cancer research. The country’s significant burden on immunological diseases creates ample opportunities for focused studies. Sponsors looking to conduct trials in these key therapeutic areas will find a well-developed infrastructure, experienced investigators, and a responsive patient population in Poland.
2. Clinical Trial Landscape in Hungary: A Hub for Innovation

Hungary stands out as a strategic location for conducting clinical trials within Central and Eastern Europe (CEE). With a population exceeding 9.8 million and a robust healthcare infrastructure, Hungary offers significant opportunities for clinical trial sponsors. As a member of the European Union, Hungary’s adherence to EU Clinical Trials Regulation further strengthens its position as an attractive destination for clinical research.
Regulatory Environment & Approval Timelines
As an EU member state since 2004, Hungary complies with the European Union Clinical Trials Regulation (EU CTR), which streamlines the approval process for clinical trials across Europe. This harmonized regulatory framework ensures consistency in ethical review and submissions, benefiting both sponsors and patients.
The National Institute of Pharmacy and Nutrition (OGYÉI) oversees clinical trial approvals in Hungary. The regulatory body’s efficiency and adherence to international standards make the approval process transparent and predictable.
Hungary’s average clinical trial approval timelines range from 60 to 90 days, depending on the complexity of the trial. This efficiency is bolstered by the country’s compliance with the EU CTR, which promotes faster and more standardized processes. Short approval timelines are crucial for sponsors aiming to accelerate study initiation and reduce costs. Hungary’s regulatory landscape ensures that trials can begin promptly while maintaining stringent safety and ethical standards.
Key Therapeutic Areas
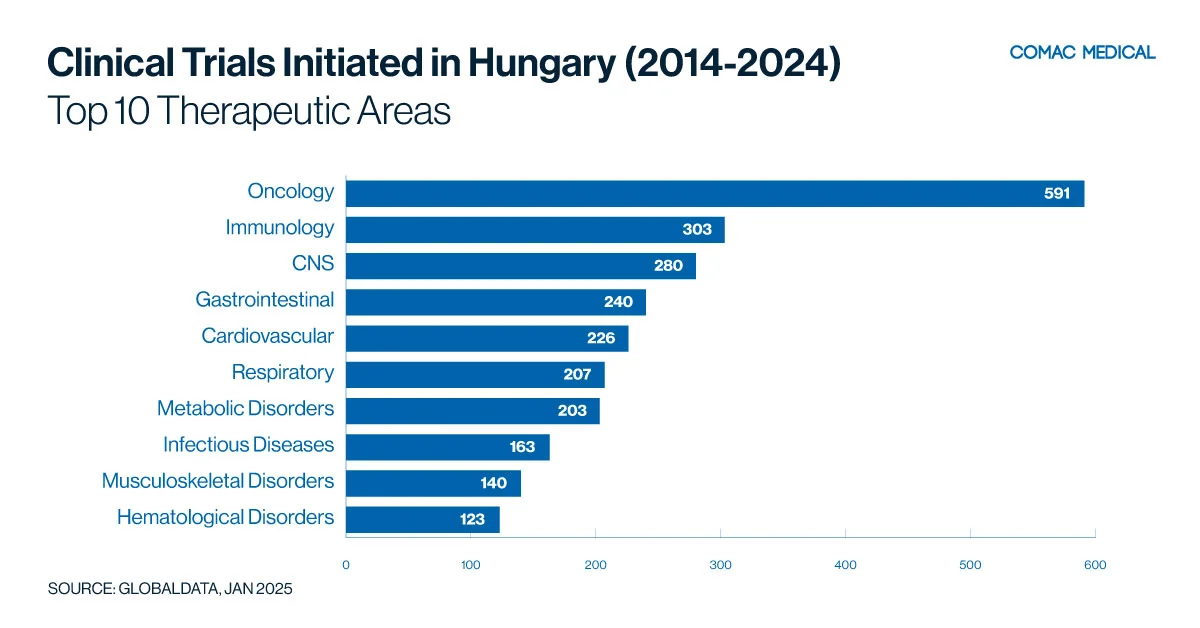
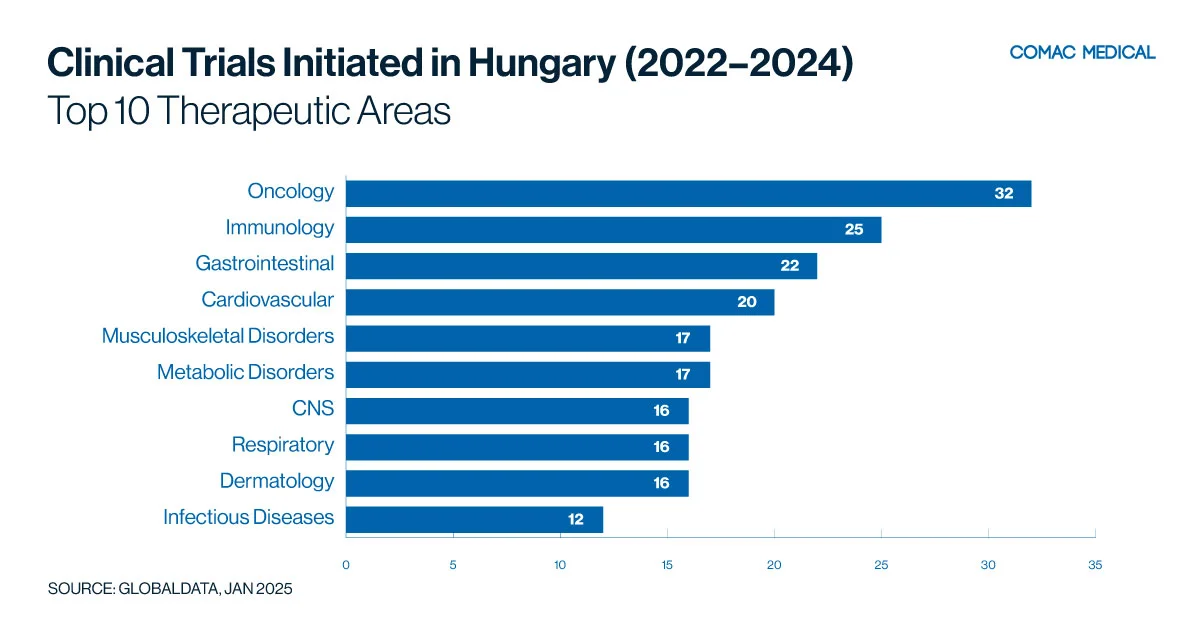
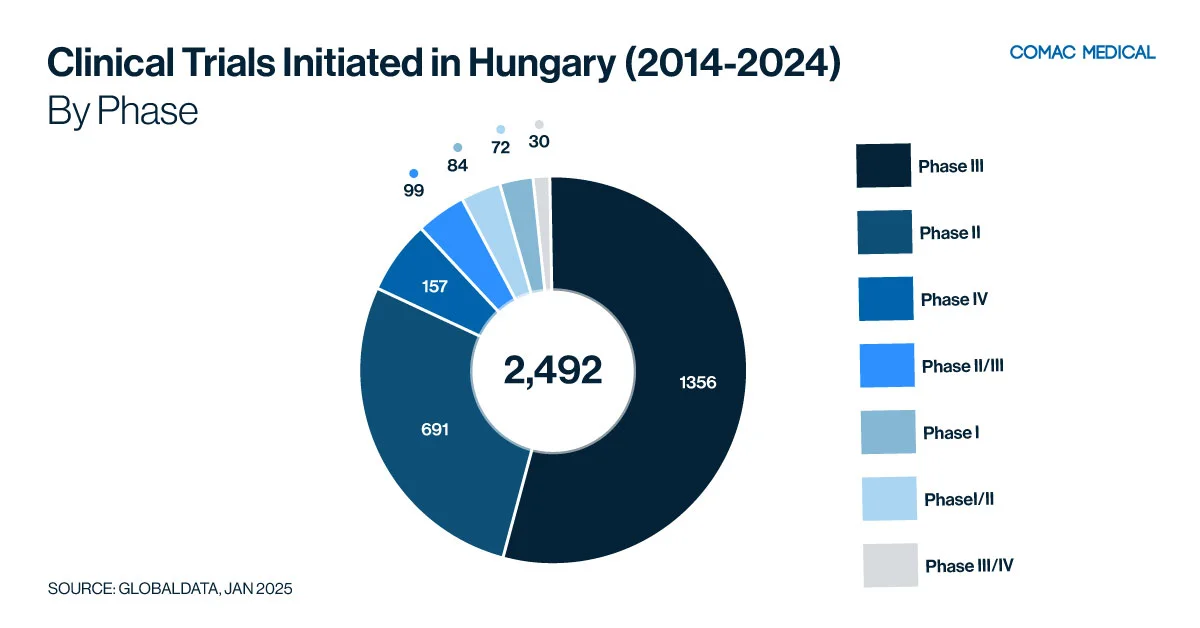
Hungary’s clinical trial landscape is particularly active in oncology and immunology, which have remained the country’s top therapeutic areas over the past decade (Figure 2(A)). This long-standing expertise highlights Hungary’s strong research infrastructure and investigator experience in these fields. In recent years, there has been a moderate increase in gastrointestinal clinical studies, further diversifying the country’s research portfolio (Figure 2(B)). The presence of specialized research centers and skilled investigators continues to enhance Hungary’s capabilities across these therapeutic areas.
3. Bulgaria: A Growing Leader in Clinical Trials in Central and Eastern Europe
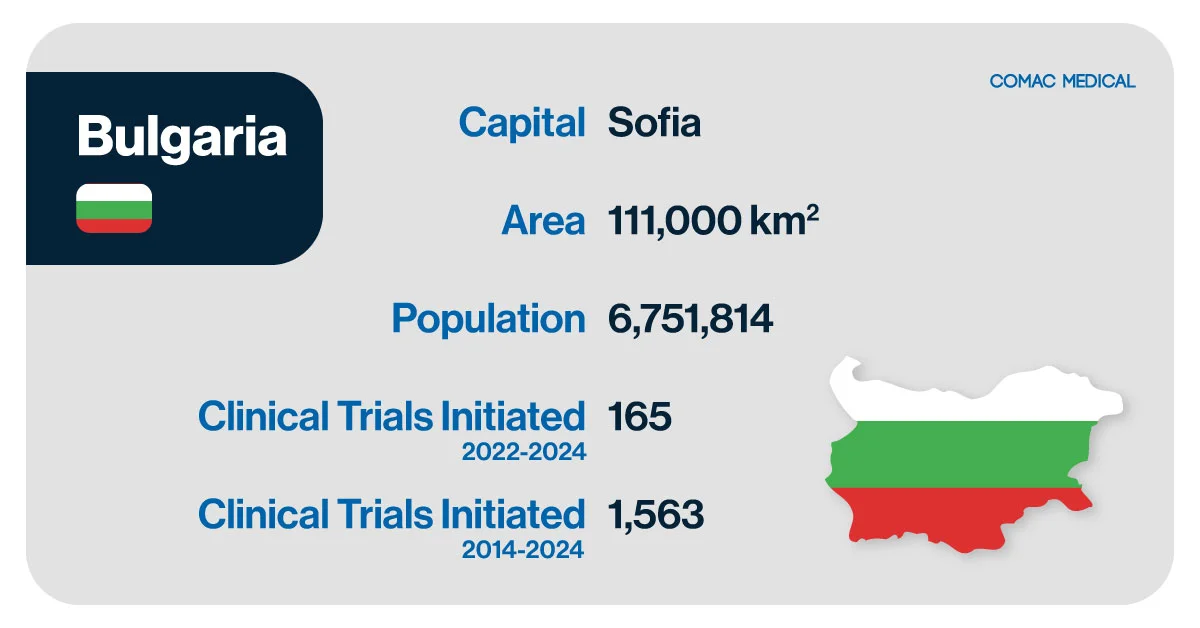
Bulgaria is a strong hub for clinical trials within Central and Eastern Europe, offering a highly skilled medical workforce and a growing reputation for excellence in research. With a population of over 6.8 million, the country combines efficient regulatory processes, competitive operational costs, and diverse patient demographics, making it an attractive destination for sponsors.
Regulatory Environment & Approval Timelines
As a member of the European Union since 2007, Bulgaria adheres to the European Union Clinical Trials Regulation (EU CTR), ensuring a streamlined and harmonized process for clinical trial approvals. The Bulgarian Drug Agency (BDA) oversees the regulatory and ethical compliance of clinical trials, offering a transparent and efficient approval system.
The implementation of EU CTR has significantly improved Bulgaria’s clinical trial landscape by providing clear timelines and standardized submission processes. The country’s adherence to EU guidelines guarantees high-quality data and patient safety, making it a reliable location for global sponsors.
Bulgaria is known for its competitive regulatory timelines, with clinical trial approvals typically completed within 60-90 days. The integration of the EU CTR framework ensures consistency and efficiency in the application process, reducing delays and enabling quicker trial initiation.
Key Therapeutic Areas
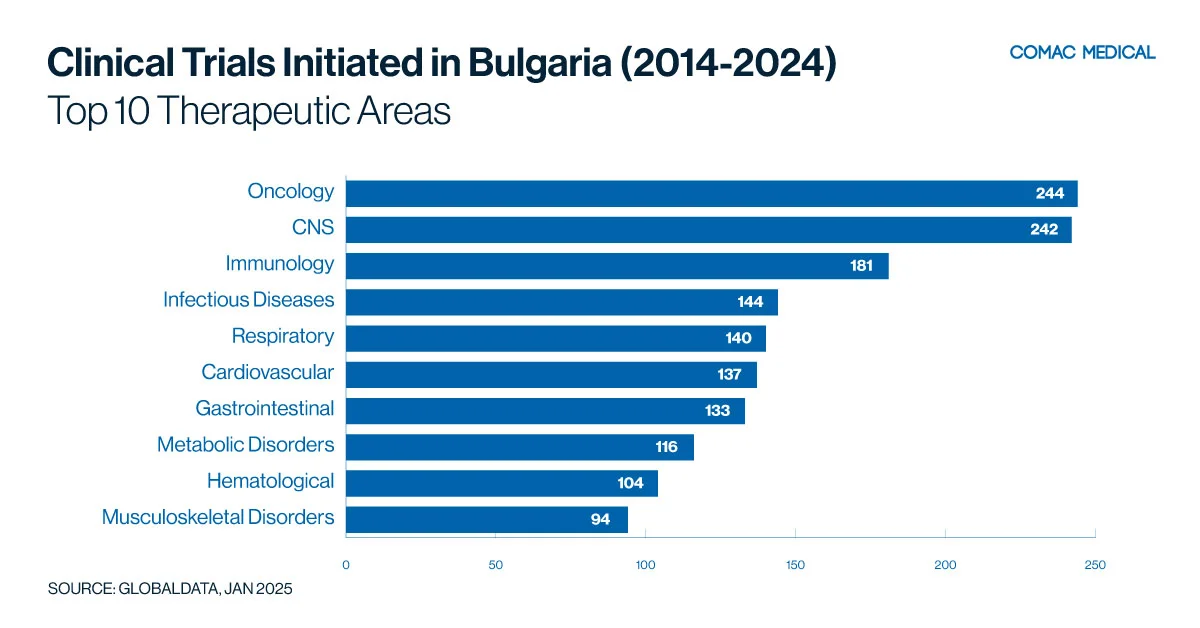
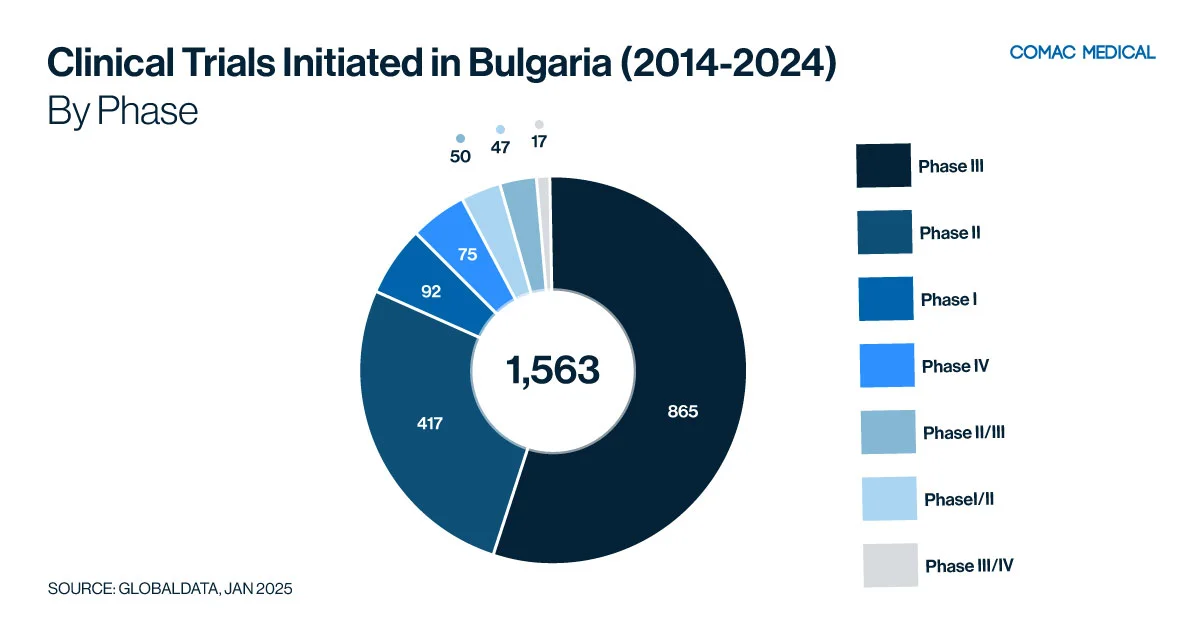
Over the past 10 years (Figure 3(A)), it is clear that Bulgaria has a strong clinical trial presence in oncology, neurology, and immunology, reflecting the country’s health priorities and disease prevalence. Oncology studies benefit from Bulgaria’s specialized cancer centers and experienced investigators. In addition, neurology is another growing focus area, driven by increasing rates of neurological disorders such as Alzheimer’s disease and multiple sclerosis.
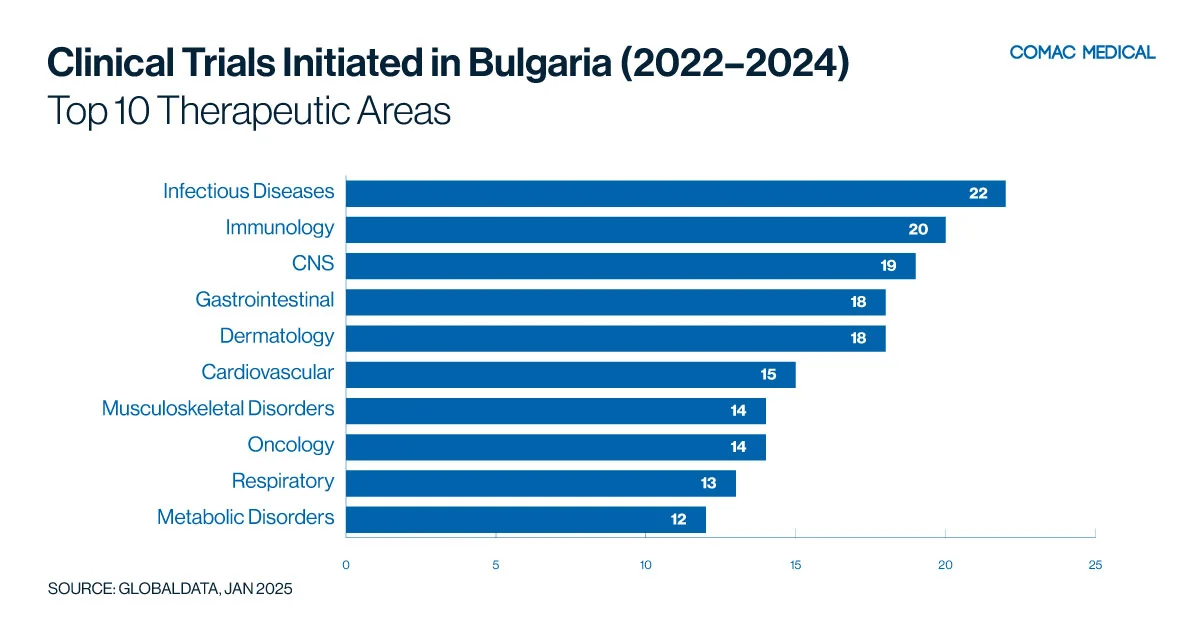
Between 2022 and 2024 (Figure 3(B)), Bulgaria experienced a notable increase in clinical trials focusing on infectious diseases, immunology, and neurology. This trend highlights the country’s robust investigator network and dedicated patient population, which together create an ideal environment for conducting high-quality research in these critical therapeutic areas.
4. Clinical Trials in Romania: A Rapidly Growing Market
Romania has emerged as a key player in the clinical trial landscape of Central and Eastern Europe, offering a combination of a large patient population and a cost-effective environment for research.
Regulatory Environment & Approval Timelines
As an EU member state since 2007, Romania adheres to the European Union Clinical Trials Regulation (EU CTR), which ensures a harmonized and streamlined process for clinical trial approvals. The National Agency for Medicines and Medical Devices of Romania (NAMMDR) is responsible for overseeing clinical trial applications and ensuring compliance with EU and national regulations.
Romania’s adherence to the EU CTR framework guarantees standardized approval processes, ethical compliance, and high-quality data. The country’s transparent regulatory system makes it a reliable partner for global clinical trial sponsors.
Romania offers competitive regulatory approval timelines, with most clinical trial applications being approved within 60-90 days. The EU CTR framework has further streamlined the process, reducing administrative burdens and expediting trial initiation. This efficiency translates into shorter start-up times, allowing sponsors to focus on the operational aspects of their trials and bringing therapies to market faster.
Key Therapeutic Areas
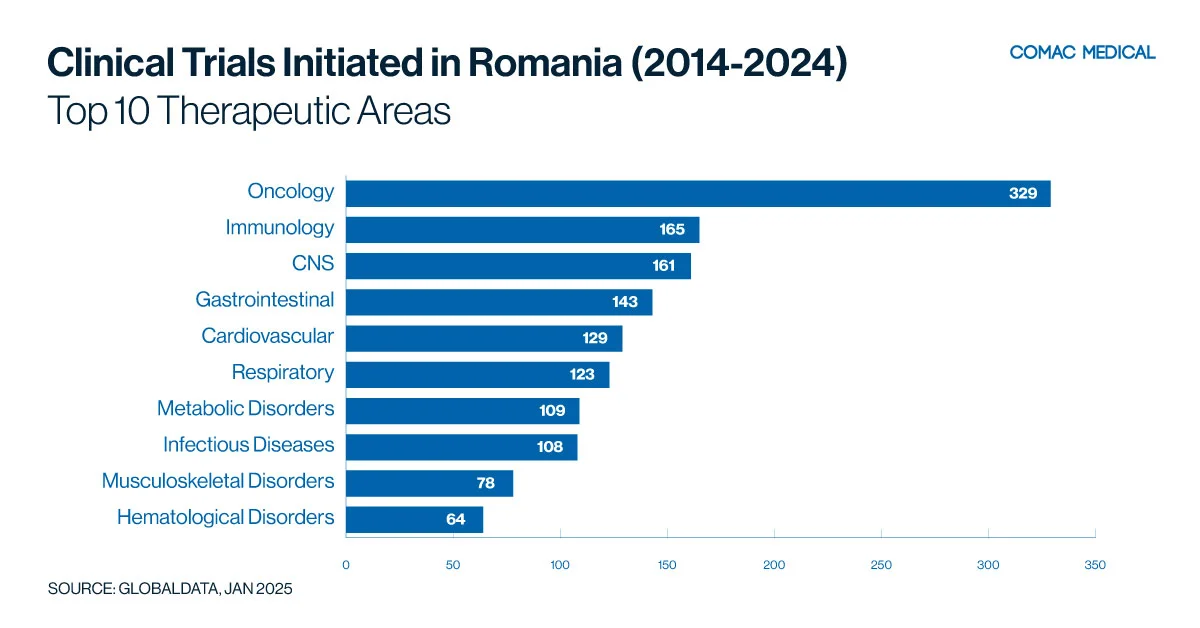
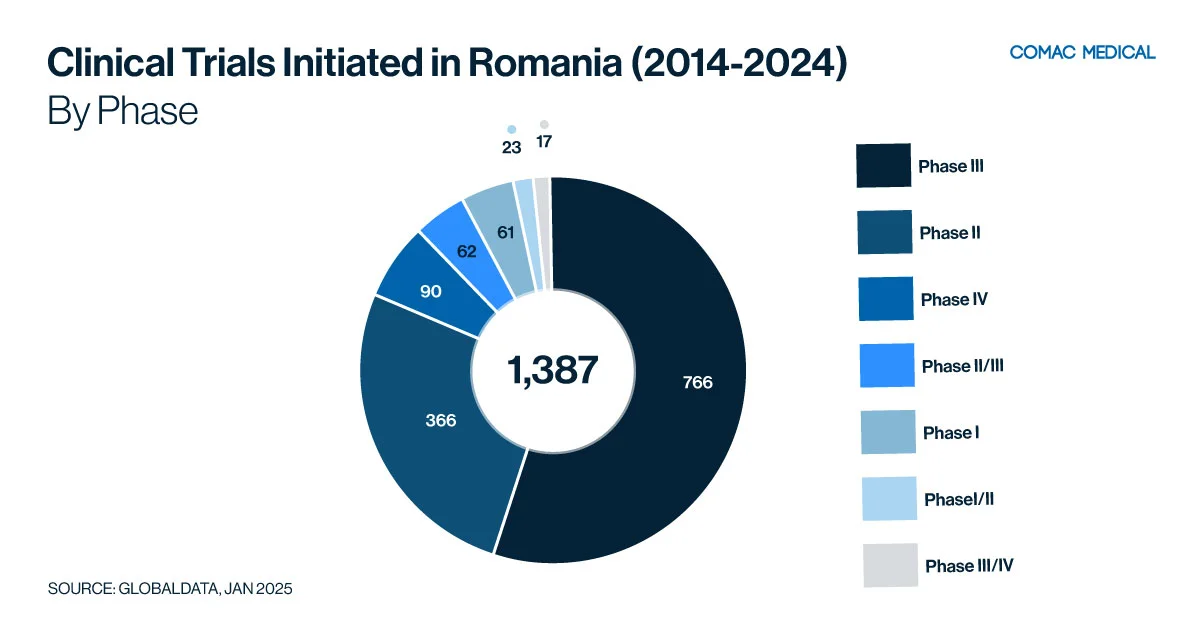
Romania has a strong presence in clinical trials across oncology, infectious disease and neurology, as demonstrated by Figure 4(A) below, which highlights the distribution of clinical trials by therapeutic area. Notably, oncology trials nearly double the number of those in the next most common therapeutic area, with 329 oncology studies compared to 165 in immunology. This significant difference underscores the strong focus on oncology research.
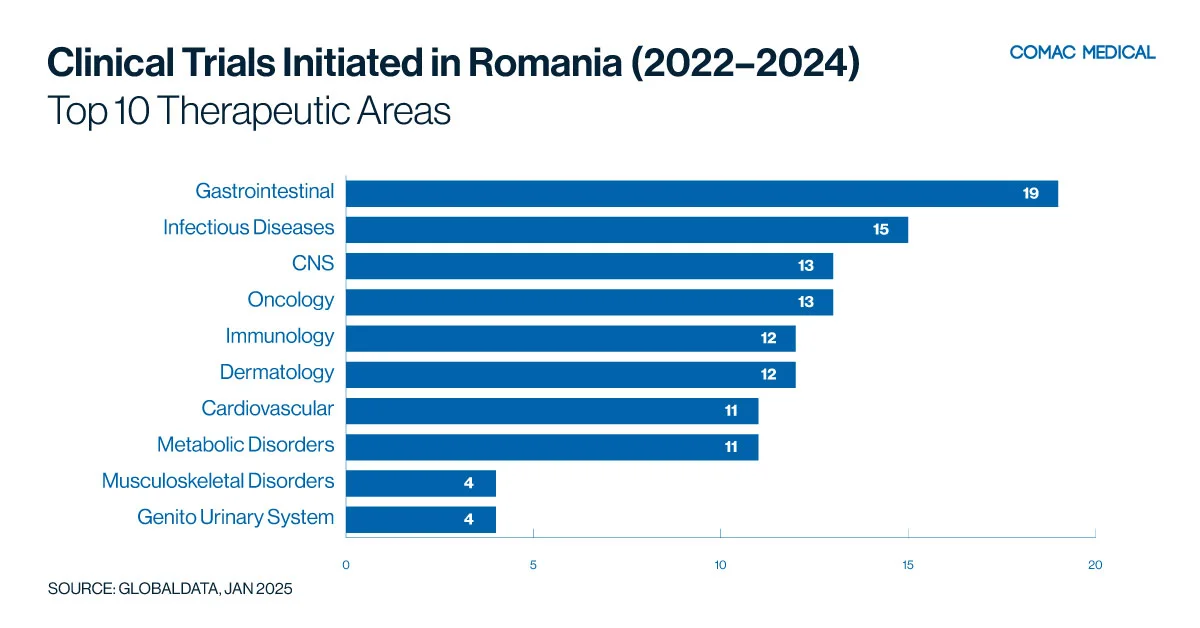
Specifically, over the past three years (January 1, 2022, to December 31, 2024), (Figure 4(C)) the country has shown the highest activity in gastrointestinal trials, followed closely by infectious diseases and neurology.
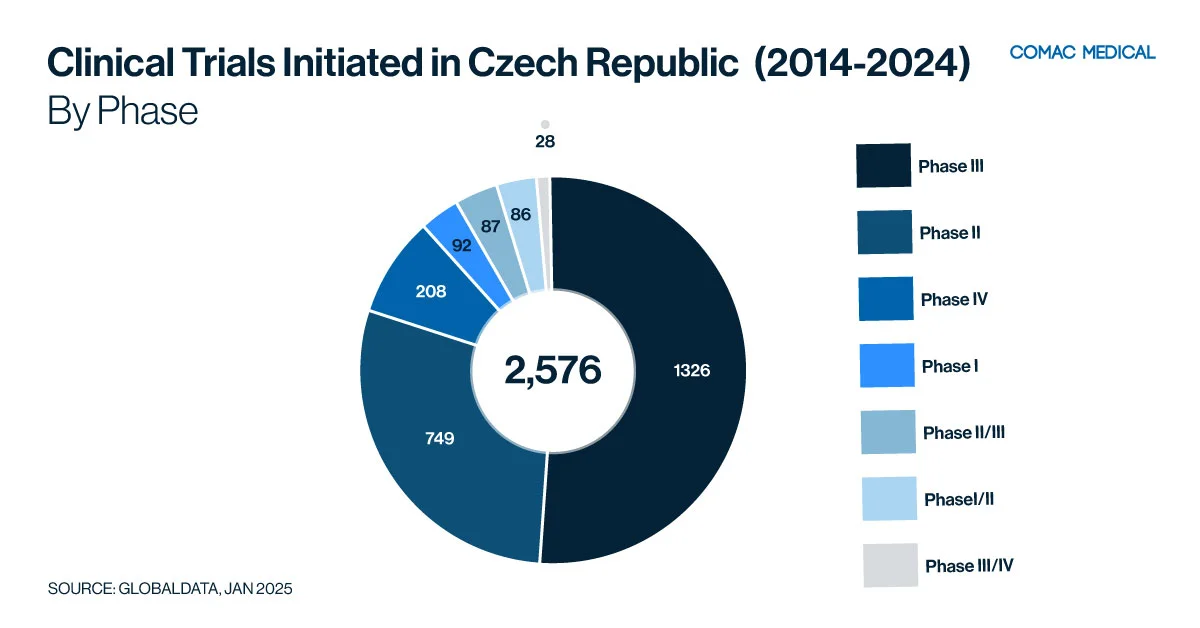
5. Czech Republic: A Strategic Location for Clinical Trials
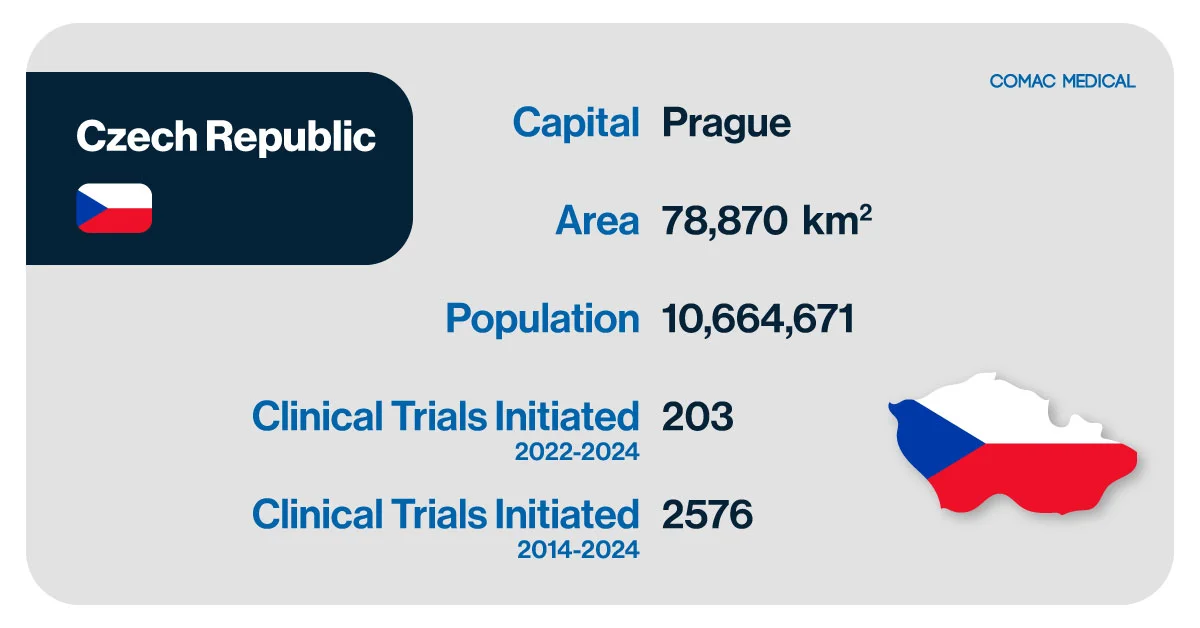
The Czech Republic has established itself as a prominent hub for clinical trials in Central Europe, offering a sophisticated healthcare infrastructure and access to highly skilled medical professionals. With a population of over 10.5 million, the country provides a well-organized system for efficient patient recruitment and quality-driven research.
Regulatory Environment & Approval Timelines
As a member of the European Union since 2004, the Czech Republic operates under the EU Clinical Trials Regulation (EU CTR), ensuring a unified and efficient framework for trial approvals. The State Institute for Drug Control (SÚKL) is responsible for overseeing regulatory compliance, ethical reviews, and patient safety, guaranteeing high standards across all stages of clinical research.
Clinical trial applications in the Czech Republic are typically processed within 60-90 days, thanks to the streamlined EU CTR process and the efficiency of national regulatory bodies. This combination of regulatory clarity and timely approvals allows sponsors to initiate trials without unnecessary delays, making the Czech Republic a competitive choice for global research initiatives.
Key Therapeutic Areas
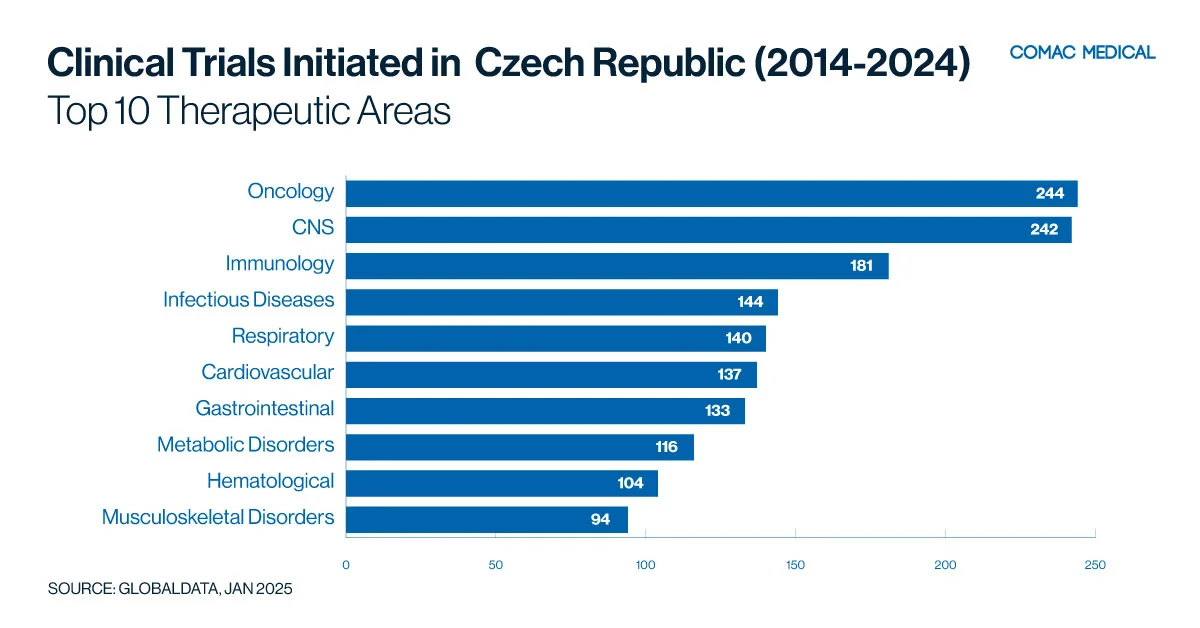
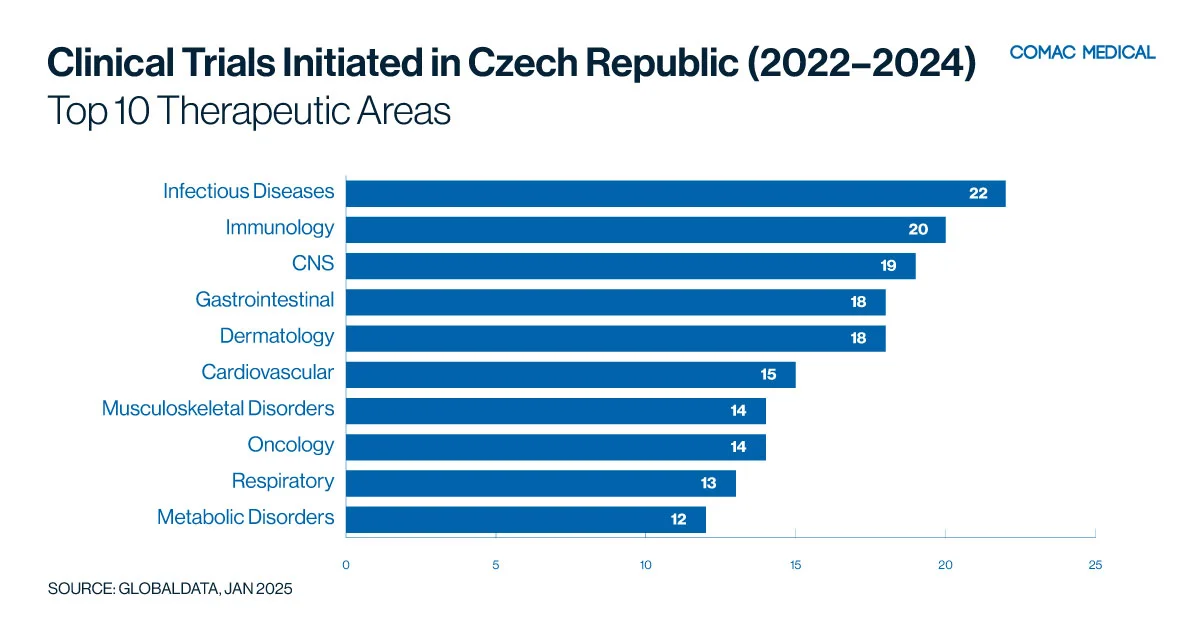
The Czech Republic has established expertise in therapeutic areas over the past decade (Figure 5(A)) such as oncology, and neurology driven by a high prevalence of related conditions and access to specialized medical facilities.
The country’s recent expertise in neurological disorders, including multiple sclerosis and Alzheimer’s disease, has also positioned it as a leader in neurology-focused clinical research (Figure 5(B)). Furthermore, the prevalence of cardiovascular diseases provides ample opportunities for studies targeting heart health, ensuring high recruitment rates and reliable outcomes.
Conclusion
Central and Eastern Europe (CEE) continues to establish itself as a premier destination for clinical trials, offering a unique blend of advantages that cater to the evolving needs of global sponsors. Across the region, countries like Poland, Hungary, Bulgaria, Romania, and the Czech Republic have demonstrated their ability to deliver high-quality clinical research through robust healthcare infrastructure, streamlined regulatory processes, and access to diverse patient pools.
Over the past decade, every country has consistently showcased its strengths in oncology research, with oncology remaining the leading therapeutic area across the region. This sustained focus reflects the high disease burden, well-developed cancer research infrastructure, and strong investigator expertise in each country.
In the past three years, distinct trends have emerged, highlighting unique areas of expertise: Poland has excelled in immunology research, Hungary has taken the lead in oncology trials, Bulgaria has gained an edge in infectious diseases, Romania has presented opportunities in gastrointestinal research, and the Czech Republic has distinguished itself in both neurology and oncology. These specialized capabilities, combined with faster recruitment rates, cost-effectiveness, and adherence to the European Union Clinical Trials Regulation (EU CTR), make the CEE region a compelling choice for sponsors.
For sponsors seeking to optimize their clinical trial strategies, CEE offers an unparalleled opportunity to reduce costs, expedite timelines, and access treatment-naïve patient populations. By leveraging the strengths of each country, sponsors can align their studies with the region’s capabilities and unlock the full potential of clinical research in this dynamic part of the world.