If your goal is to develop cost-effective alternatives to existing biological drugs (Biosimilars) or small-molecule drugs (Generics), Comac Medical offers comprehensive support and a wide range of specialized biosimilar and generics services. Our team will craft a tailored strategy to minimize risks and facilitate a smooth, cost-effective clinical development of your product.
We excel in designing and executing global biosimilar clinical plans, ensuring timely delivery and high-quality outcomes. Our services include gap analysis for comparative analytical similarity assessments, drug development consulting, and strategic advice for biosimilar and generics.
We provide clinical pharmacology expertise, bioanalytical laboratory services, and operational support for product authorization, all aimed at improving efficiency, controlling costs, and reducing risks.
Key Services:
- Design and execute global biosimilar clinical plans
- Ensure timely delivery and high-quality outcomes
- Conduct gap analysis for analytical similarity assessments
- Provide drug development consulting and strategic advice
- Offer clinical pharmacology expertise
- Pharmacokinetic and Pharmacodynamic (PKPD) support
- Deliver flexible services to improve efficiency
- Bioanalytical laboratory services
- Consulting support for product authorization
CASE STUDY
Biosimilar
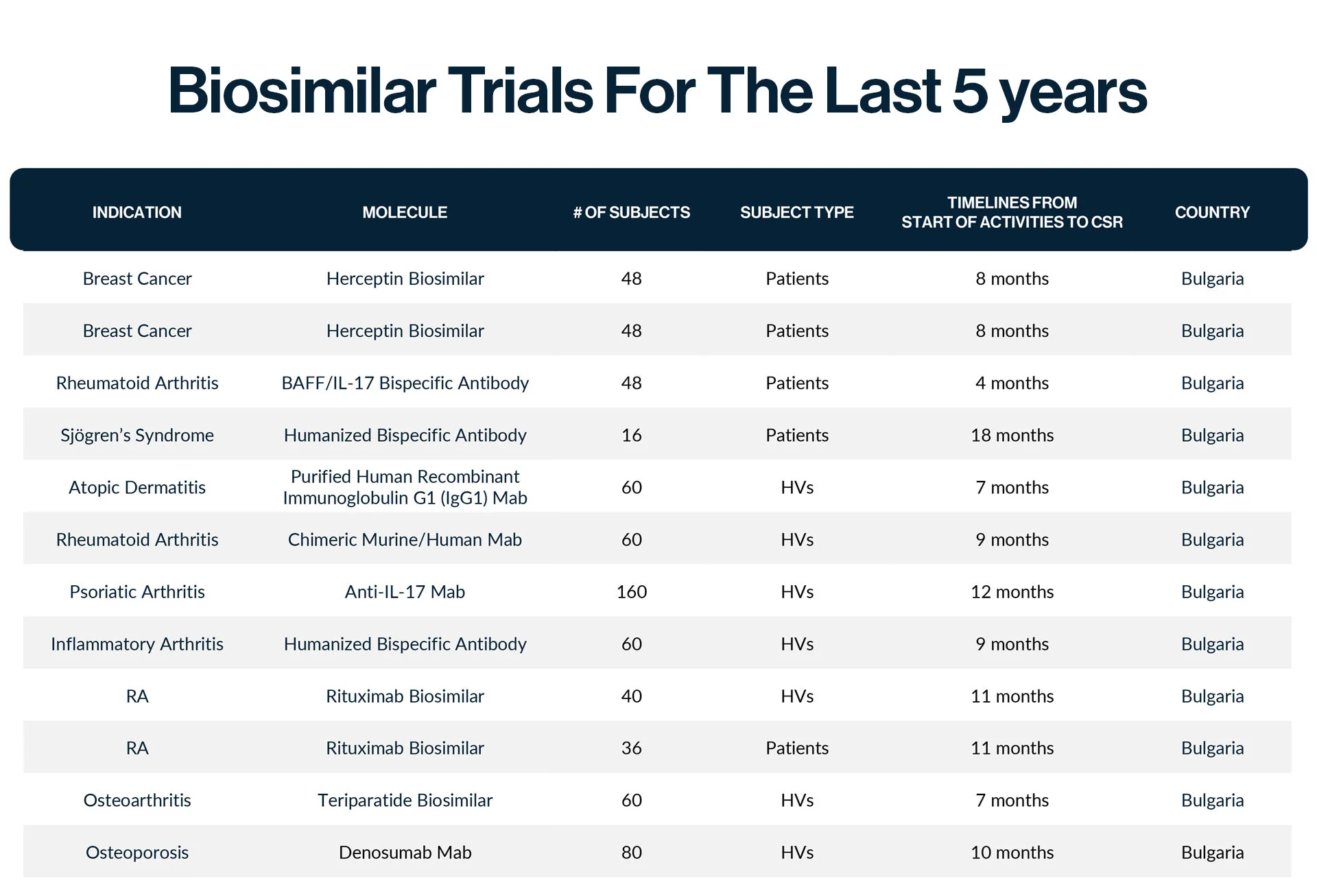
An open label, randomised, two-treatment, two-sequence, two-period, crossover study to assess the bioequivalence of proposed XXXXX biosimilar, for subcutaneous injection after single dose administration of XXXXX versus XXXXX in 160 healthy subjects.

CHALLENGES:
- Mode of administration was subcutaneous injection, which usually restricts recruitment.
- The possibility of immunogenic reactions meant that the clinical team had to be extra cautious.
- The intensive PK sampling schedule and the long hospitalization period contributed to the complexity of the study.

SOLUTIONS:
- Constant physician presence on site, experienced with various types of drug administration.
- Provision of skilled and experienced nursing staff for PK sampling.
- Very efficient planning, extensive pre-screening efforts, excellent work with subjects by the study team.
- All 160 healthy volunteers were recruited in approximately 2.5 months.
RESULTS:
Our extensive healthy volunteer database of over 4000 subjects, the efforts of our recruitment specialists, and the expertise of our highly skilled and experienced nursing and medical staff at the Phase I Unit were all factors that played in concert to ensure the success of the study. This study was an example of our ability to recruit very large numbers of subjects within very short timelines.

and Medical Progress
At Comac Medical, we are dedicated to delivering precision and fostering progress in every project we undertake.
Partner with us to experience innovative solutions and achieve unparalleled success in your clinical trials.