
Each year, on the last day of February, the world comes together to recognize Rare Disease Day, in an effort to raise awareness of the 300 million people globally who live with a rare condition. For many of these individuals, an effective treatment remains a mere hope; according to the European Federation of Pharmaceutical Industries and Associations, there have been over 7,000 rare diseases that have been identified, yet fewer than 5% have available treatments. Despite medical advancements, rare disease clinical trials face significant hurdles.
Unlike common conditions, rare diseases affect small, widely-dispersed patient populations, making it challenging to recruit participants, design effective studies, and generate the robust data needed for regulatory approval. These barriers hinder the development of essential therapies, leaving many patients with limited or no treatment options.
However, progress is being made. With the rise of orphan drug programs, regulatory incentives, and innovative trial designs, rare disease research is evolving. Organizations like Comac Medical play a crucial role in overcoming these challenges, offering expertise in patient recruitment, site selection, and regulatory navigation to accelerate rare disease clinical trials.
This article delves into the key barriers in rare disease clinical research, the impact of regulatory frameworks on drug development, while providing solutions for improving access to life-changing treatments.

1. The Key Challenges of Rare Disease Clinical Trials
Rare Disease Clinical Trials Design and Methodological Limitations
Designing clinical trials for rare diseases presents a unique challenge, as traditional randomized controlled trials (RCTs) often don’t fit the boundaries set due to small patient populations. To produce statistically significant results, RCTs rely on vast sample sizes, however, collecting sufficient robust data becomes nearly impossible when only a few hundred people worldwide are affected by a condition. The limitation of small sample size encourages researchers to reconsider the norm for rare disease clinical trial design and conduct.
Another major challenge concerns the absence of standardised outcome measures for many clinical trials in rare diseases. Unlike extensively researched conditions which have clear biomarkers and pre-defined clinical endpoints, many rare diseases may not have objective ways to characterise disease progression and assess treatment effectiveness. This creates a dilemma: should funding and time be directed toward medical laboratory research to study the disease in greater depth to configure these measures, or should clinical trials proceed with the available, and in some cases limited knowledge? The answer to this question remains unanswered and continues to spark debate within the scientific and pharmaceutical community, as both approaches are crucial to advancing rare disease treatments.
There is no doubt that medical research is essential for gaining a deeper understanding of rare diseases, including their causes, symptoms, disease progression, and underlying mechanisms. This knowledge is crucial for developing effective treatments and designing clinical trials. At the same time it should also be noted, clincial trials are also essential to test whether the treatment developed by medical research is genuinely effective and translates into real-world benefits.

Rare Disease Patient Recruitment and Retention
Rare disease patient recruitment is one of the most pressing challenges in rare disease clinical trials as traditional recruitment methods are ineffective for rare diseases. This is because these diseases affect a small number of people who are often spread across vast geographical areas, including different regions and continents.
In addition, the challenge of public recruitment for clinical trials is exacerbated by the high number of individuals with rare diseases who remain undiagnosed or misdiagnosed. Eventually, even when patients are identified, trial participation can quickly become arduous, requiring numerous hospital stays, invasive procedures, and lengthy commutes to specialised rare disease centers. These demands can be overwhelming, especially for patients already managing complex health conditions, leading to high dropout rates and incomplete trial data.
To overcome these barriers, adopting patient-centric approaches can significantly improve recruitment and retention. Strategies like utilising global patient registries, partnering with advocacy groups, and adapting rare disease clinical study design would allow seamless identification of participants, improve patient knowledge and foster better patient involvement in trials too. Integrating decentralised trial elements like remote monitoring and home-based assessments to reduce patient burden can increase speed and efficiency of patient recruitment while improving retention.

Ethical Concerns and Placebos
Ethical concerns further complicate rare disease clinical research, particularly regarding the use of placebos. In some rare diseases where no existing effective treatment is available, patients, caregivers, clinicians and advocacy groups are often reluctant to participate in placebo-controlled studies, as there is a risk of non-treatment and believe that withholding a potentially life-changing therapy, even for the sake of scientific rigor, is ethically unjustifiable.
In cases where an approved therapy already exists, some clinical trials may require participants to discontinue that treatment to be eligible for enrollment. This can lead to ethical concerns and a reluctance to participate, particularly in cases of progressive rare diseases where discontinuing an approved therapy may cause further harm.
This has led to the adoption of alternative trial models that prioritise both scientific validity and patient well-being. For example, usage of adaptive trial designs, which allow for mid-study modifications based on emerging data, making studies more efficient and responsive to patient needs. In addition, the usage of innovative trial designs such as basket trials which test a single drug across multiple rare diseases with shared molecular characteristics can expand the potential patient pool.
Meanwhile, real-world data integration, using electronic health records, patient registries, can help supplement traditional clinical trial data, providing additional insights into treatment effects. These innovative approaches are reshaping rare disease clinical research, making it more flexible, inclusive, and capable of delivering meaningful results despite the challenges.

High Costs and Financial Risks
As well as being scientifically challenging, rare diseases clinical research and drug development is financially demanding too. Unlike trials for more common conditions, clinical trials in rare disease have a high per-patient trial cost which comes from the interdisciplinary effort required to find and enroll eligible participants, extensive patient monitoring and need for specialised testing.
Rare disease clinical research funding is often constrained. This is due to a smaller pool of researchers and clinicians specializing in these conditions, coupled with a smaller patient population. This inherently limits rare disease market size, leading to lower drug demand and potential reluctance from pharmaceutical and biotech companies to manufacture and market these drugs.
For biotech and pharmaceutical companies who are investing in rare disease treatments, the financial risks can be significant. With fewer patients needing the therapy, the potential return on investment is lower compared to treatments for common conditions like cardiovascular diseases or cancer. This makes it difficult to secure funding, as many investors prioritize research with a higher probability of commercial success.
However, collaborations between governments, research institutions, and rare disease companies are helping to divide the financial burden and accelerate drug development. Nonprofit organizations and patient advocacy groups are also becoming more involved in driving innovation in this area by advocating for increased funding and policy support.
2. Regulatory Considerations in Rare Disease Clinical Trials
Differences in Orphan Drug Clinical Trial Regulations
The regulatory frameworks for orphan drug research differ from those created for more standard treatments, with policies designed to support rather than hinder rare disease clinical research. In traditional clinical trials, drug developers must navigate lengthy approval processes and large-scale studies to demonstrate safety and efficacy. However, for orphan drugs, incentives such as tax credits, market exclusivity, and fee reductions, have been introduced by regulatory agencies like the FDA and EMA to make rare disease drug development more attractive.
Moreover, to expedite access to promising treatments, regulatory agencies like the FDA and EMA also offer accelerated approval pathways, including Fast Track, Breakthrough Therapy, and PRIME. Additionally, the usage of surrogate endpoints, which are biomarkers or intermediate measures that can replace traditional clinical outcomes, is another important workaround that can facilitate and expedite rare disease clinical research. These regulatory adaptations acknowledge the unique challenges of rare disease trials, ensuring that promising therapies can reach patients faster without compromising safety.
Shorter Approval Timelines and Compassionate Use Programs
Regulatory agencies recognise the critical need for rare disease treatments for rare diseases, and have implemented initiatives such as compassionate use and expanded access programs which assist patients in obtaining experimental therapies that are still undergoing clinical development, prior to approvals. These programs allow individuals with life-threatening or severely debilitating rare diseases, who have no other treatment option.
By providing earlier access to experimental drugs, compassionate use programs balance the need for rigorous research with the immediate needs of patients while generating valuable real-world data that can support regulatory decisions and improve future trial designs.
3. How Comac Medical Supports Rare Disease Clinical Trials
Here, at Comac Medical, we understand the unique challenges of rare disease clinical trials and have built robust infrastructure to accelerate research in this complex field.
Our Services
With our reliable Principal Investigator (PI) network and tailored CRO services, we engage specialists in specific rare disease areas, ensuring access to qualified investigators and the correct eligible patient populations. Our highly accurate patient recruitment services leverage strong site relationships and advanced feasibility strategies, significantly reducing enrollment timelines. We also offer a free feasibility report, allowing sponsors to assess rare diseases clinical trial potential quickly and effectively.
Our Experience
Our state-of-the-art Early Phase Unit, where we regularly conduct a variety of rare disease clinical trials from Phase I trials including BA/BE studies, to Phase IV ensuring seamless trial execution. Backed by our experience in rare conditions such as hemophilia, hereditary angioedema, lupus, and von Willebrand disease, and many more, we bring expertise and precision to every project.
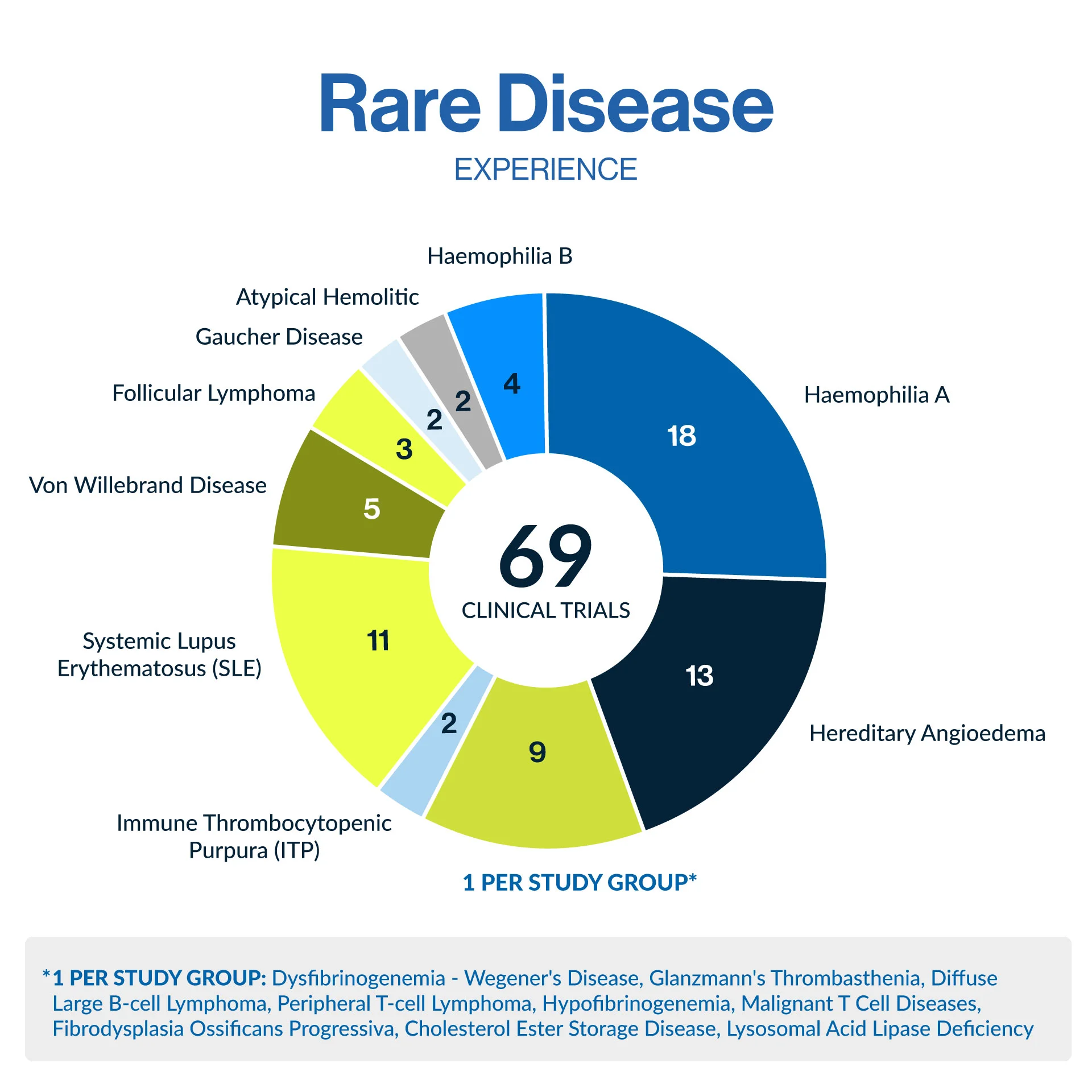
With a strong site network across Central and Eastern Europe, an emerging hub for rare disease research, Comac Medical ensures efficient trial execution while maintaining the highest regulatory and ethical standards. Our deep regulatory expertise in orphan drug regulations and approval pathways allows us to design trials that balance compliance, efficiency, and patient-centricity.
Expanding our Rare Diseases Clinical Research Portfolio
As part of our commitment to advancing rare disease research, we are expanding our portfolio by actively engaging in industry-leading events. In March 2025, we will attend the 23rd Orphan Drugs & Rare Diseases Global Congress and in November 2025 we are looking forward to attending the World Orphan Drug Congress Europe 2025. These events provide invaluable opportunities to collaborate, share insights, and further our mission of accelerating rare disease drug development.
4. A Step Forward for Rare Disease Research
Despite the challenges in rare disease clinical research, technological advancements, regulatory incentives, and growing patient advocacy are fuelling progress and investment in the orphan drug sector. Rare disease clinical trials are becoming more feasible, offering hope to millions of patients awaiting life-changing treatments.
For sponsors seeking a trusted CRO partner in rare disease clinical trials, Comac Medical offers a unique and robust blend of scientific expertise, operational excellence, and a patient-first approach to drive success in this critical area of research.



