
Clinical trial supply management is a critical function in the execution of successful clinical trials. From forecasting and packaging to trial logistics and compliance, managing clinical trial supplies requires strategic oversight, collaboration, and increasingly technological innovation. As clinical trials grow in complexity, particularly across multi-country regions like Europe, the need for streamlined supply chain processes becomes even more pressing.
The stakes are high: delays or inefficiencies in the clinical trial supply and logistics ecosystem can jeopardize study timelines, inflate costs, and risk non-compliance with regulatory requirements. According to a report by Grand View Research, the global clinical trial logistics market was valued at over USD 3.5 billion in 2022 and is expected to grow significantly, driven by increased outsourcing and the rise of decentralized trials.
This article explores the structure of clinical trial supply chain management, key operational challenges, best practices, and technological solutions that sponsors and CROs can implement to ensure efficiency and regulatory compliance, particularly in the European context.
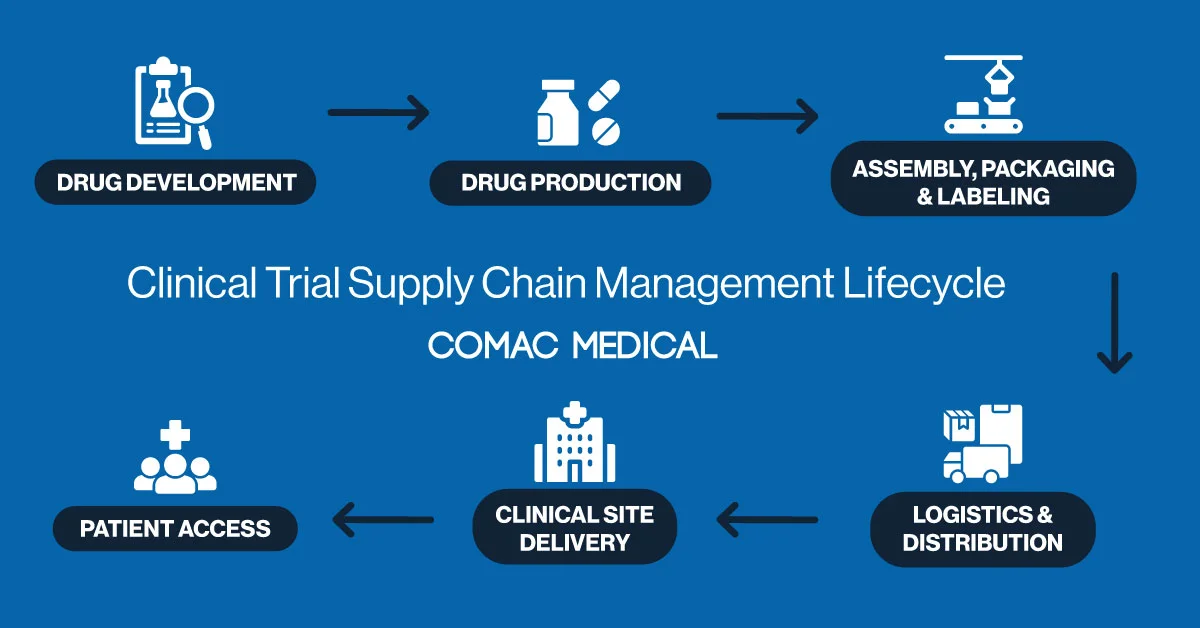
Understanding the Clinical Trial Supply Chain Management Lifecycle
The clinical trial supply chain management lifecycle is a complex, multi-phase process that must be carefully orchestrated to prevent disruptions. It begins with forecasting and planning, where supply needs are estimated based on patient enrollment projections and the specific design of the trial. This stage lays the foundation for the entire operation, setting expectations for production and delivery.
Next, the manufacturing and packaging of investigational medicinal products (IMPs) must be meticulously coordinated. Each unit must meet both study protocols and regional regulatory requirements. This is followed by labeling, a critical phase that becomes particularly nuanced in European trial operations due to the continent’s multilingual and multilayered regulatory environment. Every product label must comply with local laws while being legible and understandable to site staff.
After labeling, the focus shifts to clinical trial logistics, the movement of IMPs either to clinical sites or directly to patients. This includes cold chain transportation for temperature-sensitive therapies and route optimization for timely delivery. Finally, the cycle concludes with returns, reconciliation, and destruction of unused products, requiring precise documentation and regulatory accountability.
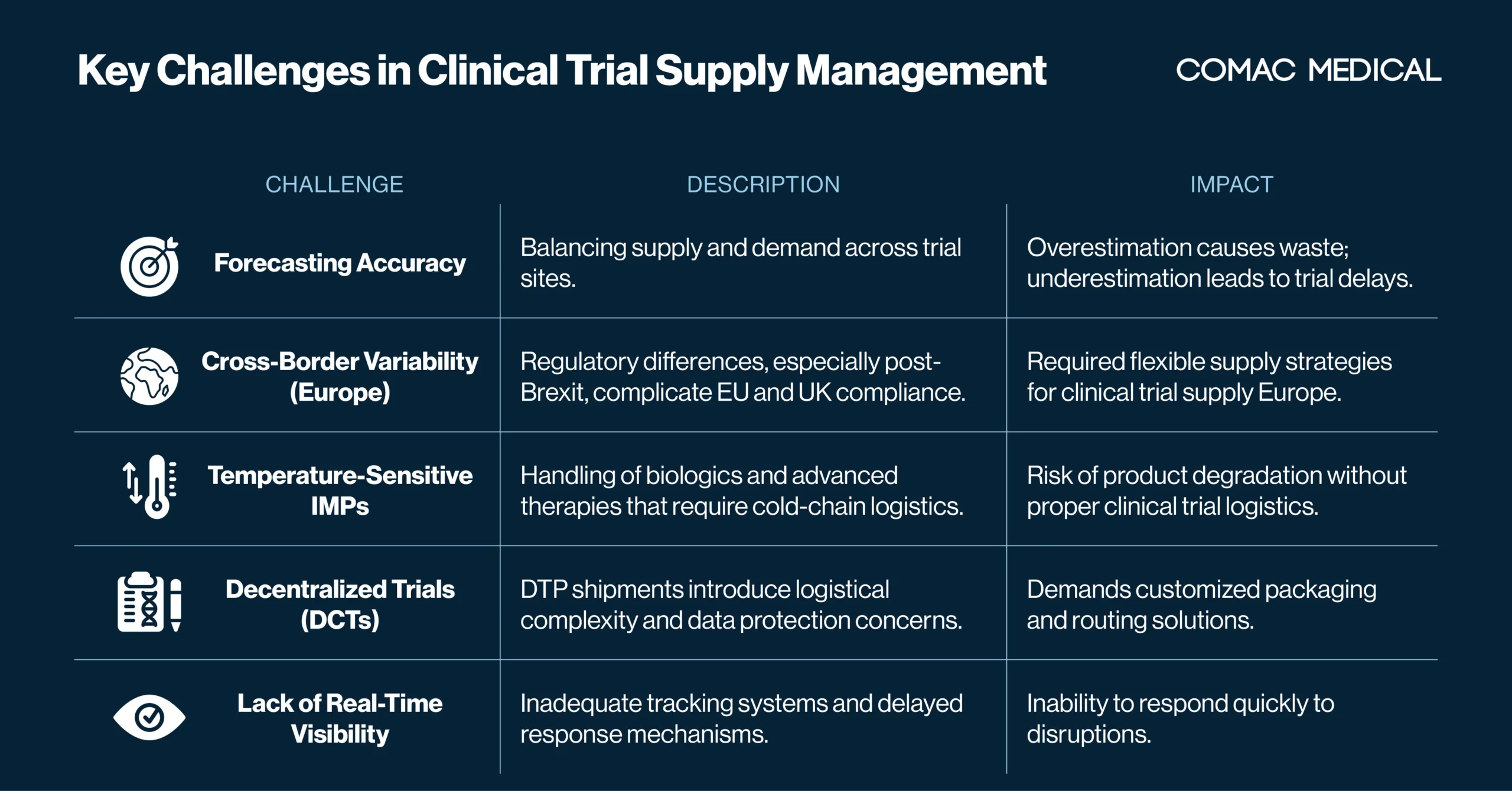
Challenges in Clinical Trial Supply Management in Europe
Despite increasing industry experience and technological adoption, trial supply planning remains burdened with challenges.
Forecasting Accuracy in Clinical Trial Supply Management
One of the most common challenges is forecasting accuracy. Overestimating demand leads to unnecessary waste and inflated costs, while underestimating supply can cause dangerous delays. This issue is particularly prevalent in European supply scenarios, where enrollment variability across countries adds additional complexity. Language barriers, recruitment fluctuations, and differing regulatory timelines often throw off projections. As a mitigation strategy, over-labeling is often used to allow flexible allocation of IMPs between countries, especially in regions outside the European Union where relabeling may not be feasible or timely due to regulatory restrictions.
To reduce risk of inaccurate forecasting accuracy, organizations are turning to predictive analytics that incorporate real-time site activation data, patient recruitment trends, and protocol amendments to dynamically adjust supply estimates. However, without a robust clinical trial supply management system, this level of accuracy is difficult to achieve, particularly for studies with rolling enrollment or multiple arms.
Managing Cross-Border Regulatory Variation in Clinical Trial Supply
Cross-border regulatory fragmentation is another persistent obstacle in clinical supply operations, especially in European studies. Post-Brexit, the United Kingdom follows separate trial submission processes from the EU, as outlined in the EU Clinical Trials Regulation (CTR). This divergence has created complications for global sponsors and CROs attempting to run pan-European trials.
Supply strategies must now account for dual compliance tracks—one for the EU and another for the UK. Inconsistent timelines, varying import/export regulations, and evolving labeling requirements mean that a one-size-fits-all approach is no longer viable. These challenges highlight the growing need for flexible yet compliant clinical trial supply chain management solutions that can adapt to regional regulatory frameworks without compromising efficiency.
Working with experienced European logistics partners and local depots can help navigate this complexity, but ultimately, technology-enabled visibility and region-specific planning are essential.
Cold Chain Management of Temperature-Sensitive IMPs
Temperature-sensitive investigational medicinal products (IMPs), particularly biologics, cell and gene therapies, and other advanced therapeutics, require highly specialized handling and constant environmental monitoring.
Ensuring that these products remain within specified temperature ranges (often between 2–8°C or even colder) from production to administration is one of the most critical components of clinical trial logistics. Even minor deviations can result in product loss, protocol deviations, or safety risks to patients.
Cold chain failures are especially challenging in remote or multi-site trials, where last-mile delivery can vary widely. Smart packaging, GPS-enabled trackers, and real-time monitoring systems are becoming industry standards in maintaining chain-of-custody and ensuring compliance. However, without proactive integration into a centralized supply management system, cold chain issues can go undetected until it’s too late.
Decentralized Clinical Trials and Direct-to-Patient Supply Challenges
The rise of decentralized clinical trials (DCTs) has transformed how clinical trial supply and logistics are approached. While DCTs offer patient-centric benefits such as increased accessibility and retention, they introduce new layers of operational complexity.
Direct-to-patient (DTP) shipments mean investigational products must be delivered to patients’ homes, sometimes across borders, often in low-volume, high-frequency batches. This shift demands new packaging configurations, temperature control methods, and compliance with home-use labeling standards. In some jurisdictions, home delivery is still under regulatory scrutiny, adding to the logistical puzzle.
Sponsors and CROs need to ensure that they not only meet delivery timelines but also maintain product integrity and data privacy, often without having on-site staff. The evolution of DCTs is pushing the boundaries of traditional supply management models, requiring innovative tools and agile infrastructure.
Real-Time Visibility and Tracking
A final yet fundamental issue in clinical trial supply chain management is the lack of real-time visibility. Without transparent tracking tools that provide live data on inventory levels, shipment progress, and site usage, sponsors and CROs are flying blind. Delays in responding to shipment failures, overages, or site stockouts can result in enrollment pauses, regulatory noncompliance, and increased costs.
Investing in integrated supply dashboards, IRT (Interactive Response Technology) platforms, and end-to-end tracking systems enables organizations to detect and respond to disruptions faster. These tools are now essential for modern supply management systems, particularly as trials become more global, complex, and decentralized.
Leveraging Technology to Improve Clinical Trial Logistics
To address growing operational challenges, many life sciences organizations are turning to advanced supply management platforms. These systems offer features ranging from forecasting and inventory tracking to integrated logistics coordination and analytics dashboards.
CTMS: Expanding Beyond Oversight
Clinical Trial Management Systems (CTMS) remains the backbone of many trial operations. Although traditionally focused on trial oversight, modern CTMS platforms are increasingly integrating supply tracking capabilities, offering a holistic view of trial performance.
IRT Systems: Automation & Real-Time Control
Interactive Response Technology (IRT) systems provide further support by automating randomization, managing site inventory, and providing real-time shipment updates. These systems are essential for maintaining balance in complex, multi-arm trials and enable immediate adjustments when needed.
Tailored Supply Chain Platforms for Clinical Trials
Specialized logistics-focused platforms such as those offered by Suvoda, Almac, or 4G Clinical go even further by offering tailored platforms focused solely on clinical trial logistics and compliance. These systems improve efficiency, reduce manual entry errors, and provide audit-ready data trails.

Best Practices for Seamless Clinical Trial Supply and Logistics
Improving clinical trial supply management and logistics requires not only the right tools but also strategic planning and interdepartmental collaboration. Below are six interconnected best practices that form the foundation of an efficient and compliant trial supply process.
Early Involvement in Protocol Design
One foundational best practice is to involve supply teams during protocol development. This early engagement ensures that the trial’s logistical feasibility is aligned with its scientific objectives. By incorporating supply considerations during protocol design such as packaging timelines, depot availability, and distribution complexity, sponsors and CROs can avoid late-stage delays and costly amendments.
Predictive Analytics and Forecasting
Advanced analytics can help sponsors and CROs fine-tune supply forecasts by factoring in historical data, current recruitment trends, and geographical variations. Predictive modeling enhances accuracy and minimizes waste, especially in multinational trials where site activation and enrollment patterns can be unpredictable. When embedded into a robust clinical trial supply chain management solution, these insights enable smarter decision-making throughout the trial lifecycle.
Cross-Functional Collaboration
Effective supply management relies on close collaboration between clinical operations, data management, regulatory, and logistics teams. Cross-functional planning ensures alignment between protocol design, recruitment strategy, and real-world logistics. It also enables faster escalation and resolution of supply chain issues, reducing the risk of site disruptions or patient dosing delays.
Vendor Partnerships and Logistics Expertise
Another essential strategy is to collaborate closely with third-party logistics providers who specialize in European trial supply. These partnerships provide access to regional depots, local expertise, and cold-chain infrastructure while ensuring compliance with country-specific regulatory nuances. Experienced vendors also support rapid deployment of IMPs and mitigate customs or import/export delays.
Performance KPIs and Quality Audits
Routine performance audits and vendor assessments are critical to maintaining high standards. Supply chain teams should continuously evaluate their service providers using KPIs, SOP adherence, and quality metrics. Metrics such as on-time delivery, temperature excursion rates, and reconciliation accuracy help identify performance gaps and support continuous improvement.
Automated Label Management Systems
Automated label management tools help reduce the complexity of language translations, regional variations, and version control across global trials. These systems streamline production and ensure that all investigational products meet local regulatory requirements before release. Incorporating label management into your clinical trial supply management system can significantly cut production timelines and regulatory risk.
Sustainability in Clinical Supply Chain
Sustainability is another key trend. Many companies are evaluating how to reduce the environmental impact of their clinical trial supply chain management by minimizing packaging waste and optimizing shipping routes. Trials involving personalized medicine add a further layer of complexity, as custom therapies demand highly agile, responsive supply strategies.
Conclusion
As clinical trials become more complex and globalized, robust clinical trial supply management has never been more essential. From forecasting and packaging to labeling, shipment, and reconciliation, every component must be optimized to ensure both efficiency and regulatory compliance.
In the context of clinical trial supply in Europe, the stakes are especially high. Sponsors and CROs must navigate a web of country-specific regulations, logistical challenges, and evolving patient expectations. By investing in smart technologies, cultivating strategic partnerships, and adopting flexible supply models, they can stay ahead of disruptions and bring innovative therapies to the market faster.
If your organization is seeking to streamline clinical trial supply and logistics, now is the time to evaluate new systems and strategies. A well-orchestrated supply chain isn’t just an operational necessity — it’s a strategic advantage.
If you’re seeking support with supply planning or want to explore innovative supply chain management solutions, get in touch with our team to learn how we can help.



