
Selecting the most appropriate clinical trial design is of utmost importance, and it can be the difference between success and failure of a clinical study. The type of clinical trial design chosen shapes every single aspect of the study, from feasibility and patient recruitment to cost, duration while adhering to regulatory requirements. Most importantly, clinical trial design directly impacts the quality of results and conclusions of the study and ultimately, the data integrity too.
As the global clinical research landscape becomes more complex and competitive, understanding various clinical trial study design options is essential for sponsors looking to bring new treatments to market efficiently.
In this article, we explore the full spectrum of the different types of clinical trial designs, from foundational designs like parallel and crossover trials to more complex and flexible approaches such as adaptive, umbrella, basket, and platform trial design. We also examine the growing role of decentralized clinical trials and pragmatic trial design, particularly in response to the increasing demand for real-world evidence and patient-centric approaches.
Whether you’re a biotech company preparing to launch your first trial, or a seasoned clinical operations professional looking to optimize study execution, this guide aims to provide a comprehensive overview to build on your current knowledge while supporting informed decision-making.
The Importance of Clinical Trial Study Design
Before diving into specific types of clinical trial designs, it’s important to understand why clinical trial study design is so critical. A well-constructed design ensures that a trial generates scientifically valid, ethically sound, reproducible, and regulatory-compliant data.
Some key factors that influence clinical trial design include:
✔️ Target patient population size and availability
✔️ The nature and variability of the disease
✔️ Expected treatment effects
✔️ Ethical considerations
✔️ Regulatory considerations
✔️ Time and budget constraints
✔️ Infrastructure availability in the target region (e.g., Central and Eastern Europe)
Equally important is the objective of the trial itself. Whether the goal is to assess efficacy, safety, or the risk-benefit ratio, the clinical trial methodology must be tailored to achieve that purpose. For instance, many studies aim to demonstrate superiority, where the new drug or intervention must prove to be more effective than the standard of care. Other studies are designed as non-inferiority trials, where the objective is to show that the test drug is not unacceptably worse than the comparator. These different goals carry distinct statistical requirements and power calculations, which in turn dictate the most appropriate clinical trial study design. The trial’s success depends on how well the design aligns with its intended outcomes.
In addition, the role of statistics in clinical trial design cannot be overstated. As the renowned statistician R.A. Fisher once warned:
“To call in the statistician after the experiment is done may be no more than asking him to perform a post-mortem examination—he may be able to say what the experiment died of.”
This insight underscores the importance of integrating statistical planning into the earliest stages of trial development. Sound statistical design ensures that the trial can generate reliable conclusions, avoid bias, and meet regulatory expectations.
In selecting the right trial design, sponsors must carefully balance scientific objectives with operational realities. By working with an experienced CRO like Comac Medical, sponsors gain a strategic partner capable of guiding design choices that maximize data quality, cost efficiency, and clinical trial feasibility, particularly in specialized or emerging markets.
Traditional Types of Clinical Trial Designs
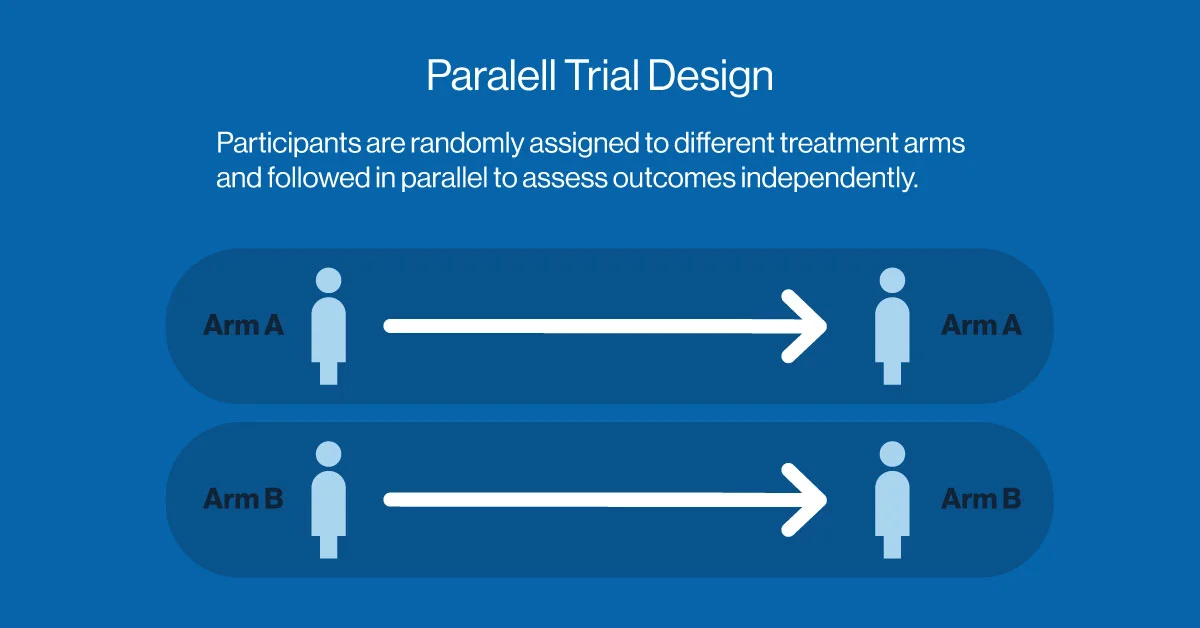
Parallel Trial Design: The Gold Standard of Simplicity
The parallel trial design is one of the most commonly used structures in clinical research. In this model, participants are randomly assigned to one of two or more groups, with each group receiving a different intervention (or placebo). The treatments are administered concurrently, and outcomes are measured in parallel.
This design is ideal for larger patient populations and conditions where treatments are expected to produce measurable effects over a relatively short period. It minimizes bias and simplifies statistical analysis, making it a preferred option for confirmatory (Phase III) studies. However, a notable challenge is the risk of loss to follow-up, where participants become uncontactable or drop out. This can introduce attrition bias, reduce the effective sample size, and potentially limit the generalizability of the trial findings.
However, its simplicity comes at a cost: parallel trials typically require larger sample sizes than other models, which may extend timelines and increase costs. Despite this, it remains a cornerstone of clinical trial methodology, particularly in the pharmaceutical development pipeline.
Crossover Trial Design: Efficiency Through Within-Subject Comparison
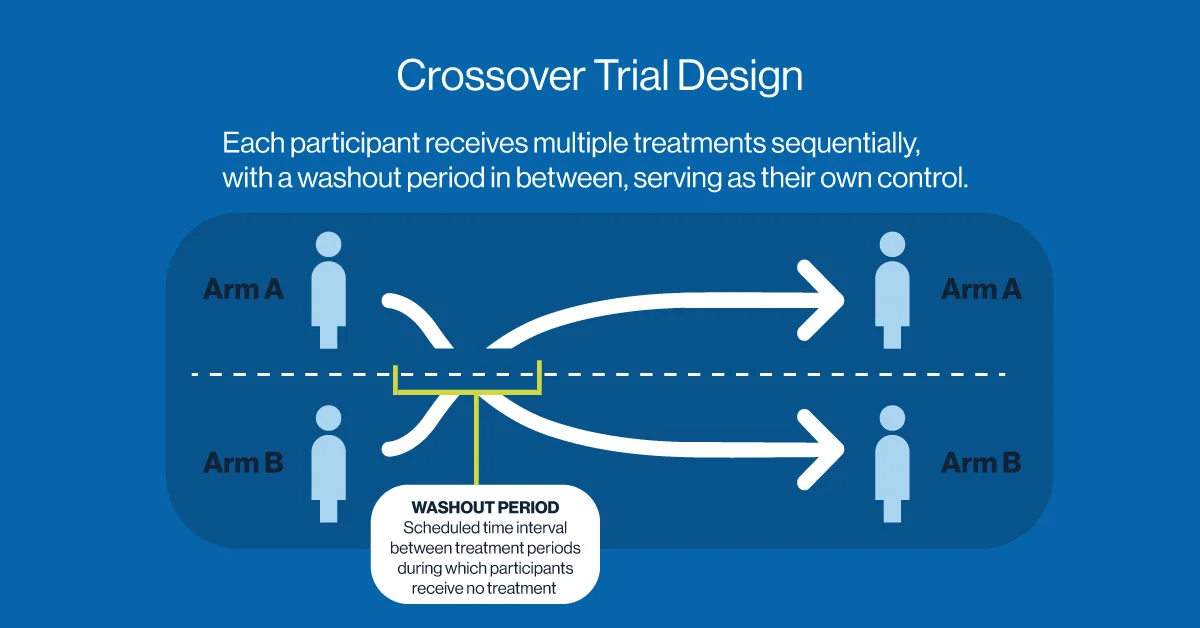
The crossover trial design offers an efficient alternative, particularly for chronic conditions or stable diseases. In this approach, participants receive multiple treatments sequentially, serving as their own control. After a washout period, they “cross over” from one treatment to another, allowing for direct within-subject comparisons.
This format is statistically powerful and often requires fewer participants. It’s frequently used in studies involving pain management, metabolic disorders, or behavioral interventions.
However, crossover designs are not suitable for all conditions. They rely on the assumption that treatment effects do not persist beyond the washout period. If residual effects from the first treatment influence the outcomes of subsequent treatments, carryover bias can occur which may potentially distort results and undermining the validity of comparisons. This limits the applicability of crossover designs in conditions involving irreversible disease progression or long-acting therapeutic effects.
Innovative and Complex Clinical Trial Designs
Adaptive Trial Design
Unlike traditional fixed designs, adaptive trial design allows for prespecified modifications to the trial based on interim data. These adaptations can include changes in sample size, patient allocation, trial duration, treatment arms, dose level or even endpoint selection—without compromising the study’s integrity or validity.
Adaptive designs offer the advantage of real-time decision-making, enabling sponsors to stop trials early for efficacy or futility, or to add promising new arms. They can significantly reduce costs and timelines while improving the chances of identifying effective therapies.
However, adaptive trials require sophisticated planning, statistical modeling, and frequent data monitoring. Regulatory agencies such as the FDA and EMA have issued guidance to support their implementation, but execution requires close collaboration between biostatisticians, data managers, and CROs.
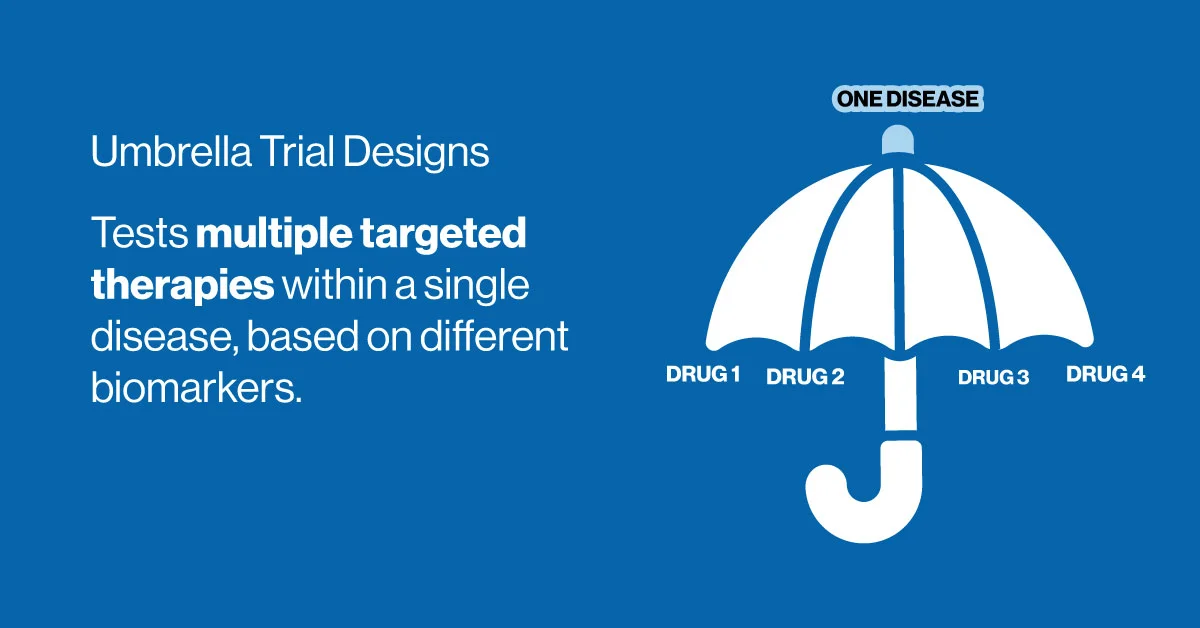
Umbrella Trial Design
As precision medicine advances, the types of clinical trial designs have had to evolve to accommodate the complexity of molecular targeting. The umbrella trial design addresses this need by testing multiple therapies within a single disease population, stratified by biomarkers or genetic profiles.
For example, in a lung cancer umbrella trial, patients with different mutations (e.g., EGFR, KRAS, ALK) might be assigned to separate arms, each testing a different targeted agent. This approach allows researchers to evaluate multiple hypotheses simultaneously under a shared protocol.
While umbrella trials offer efficiency and flexibility, they require significant coordination and regulatory alignment, particularly around biomarker testing and adaptive features.
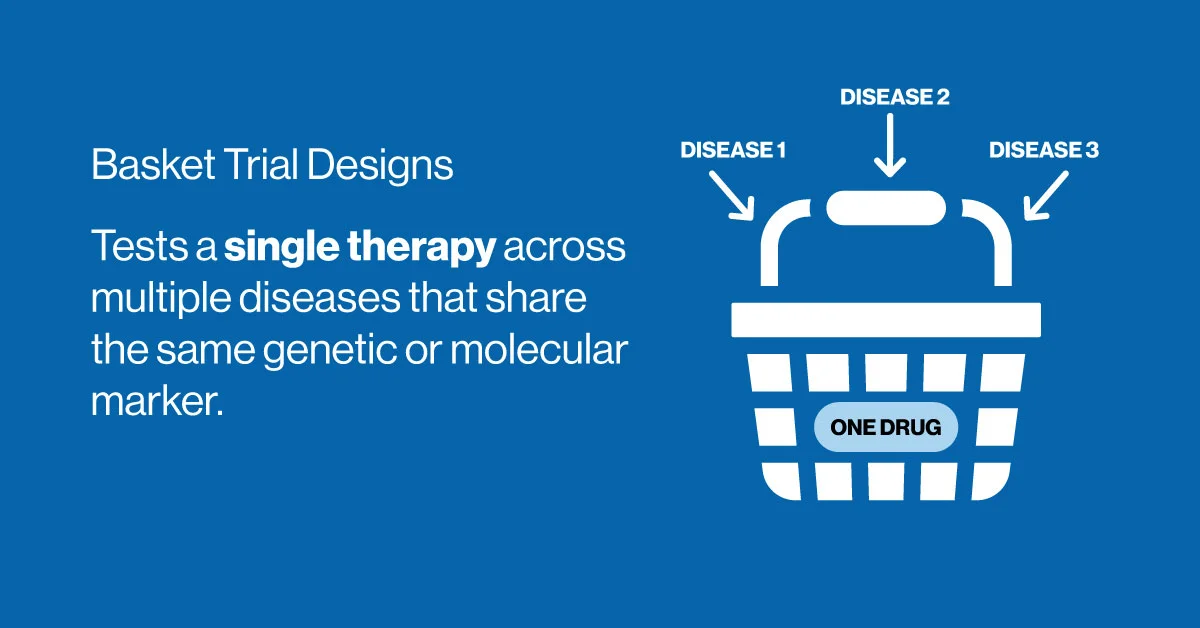
Basket Trial Design
In contrast to umbrella trials, the basket trial design tests a single therapy across multiple diseases that share a common molecular feature. For instance, a drug targeting a specific gene mutation may be evaluated in patients with breast, lung, and colon cancers, provided that all tumors express the relevant biomarker.
This design is especially powerful for investigating targeted therapies and has gained popularity in early-phase oncology research. It enables rapid hypothesis testing and expands therapeutic potential across indications.
One challenge with basket trials is the statistical analysis of heterogeneous patient populations, which may vary widely in response and disease progression. Still, when executed correctly, basket trials offer a cost-effective, innovative pathway to demonstrating proof of concept.
Platform Trial Design
The platform trial design represents a paradigm shift in clinical research. Unlike traditional trials that begin and end with a fixed protocol, platform trials are perpetual studies with a master protocol, allowing treatments to enter or exit the trial based on ongoing results.
These trials are particularly valuable in areas where therapeutic development is dynamic, such as infectious diseases, oncology, and neurology. One prominent example is the I-SPY 2 breast cancer trial, which evaluates multiple investigational drugs in a seamless structure.
Platform trials offer unmatched flexibility and resource efficiency. They reduce downtime between studies and eliminate the need to launch separate trials for each intervention. However, they demand robust infrastructure, regulatory coordination, and experienced oversight, areas where CROs with operational depth like Comac Medical can play a key role.
Modern Approaches: Pragmatic and Decentralized Trials
Pragmatic Trial Design
While traditional trials focus on efficacy under controlled conditions, pragmatic trial design emphasizes effectiveness in real-world clinical practice. These studies typically include broader patient populations, less stringent inclusion/exclusion criteria, and rely on routine healthcare settings.
The goal is to evaluate how a treatment performs in day-to-day practice, offering insights that complement the findings of traditional RCTs. Pragmatic trials are increasingly used by payers and health authorities seeking to assess the real-world value of interventions.
Implementing a pragmatic trial requires careful balance. Design must preserve scientific rigor while embracing variability. Key elements include integrating electronic health records, patient registries, and sometimes decentralized components.
Decentralized Clinical Trials
The rise of digital technologies and remote monitoring has paved the way for decentralized clinical trials (DCTs), in which some or all study procedures are conducted outside of traditional research sites. DCTs may include eConsent, home nursing visits, telemedicine, and wearable device monitoring.
By reducing the need for site visits, DCTs improve patient access, reduce dropouts, and increase geographic diversity. They are particularly effective in rare d
isease trials, pediatrics, and hard-to-reach populations.
Despite their promise, DCTs present challenges in regulatory acceptance, technology integration, logistical complexity, and data quality. Partnering with a CRO familiar with both traditional and decentralized models ensures a smoother transition and stronger oversight.
Choosing the Right Clinical Trial Design: A Strategic Decision
Selecting the optimal clinical trial study design is more than a scientific decision—it’s a strategic one. It requires understanding the target indication, anticipated treatment effect, and operational realities in various markets.
Sponsors must also consider:
✔️ Clinical trial feasibility in selected geographies
✔️ Available infrastructure for adaptive or decentralized models
✔️ Regulatory pathways and local ethics board expectations
✔️ Timelines for enrollment and data collection
✔️ Budget and resource availability
At Comac Medical, we work with sponsors from early protocol development through execution, offering guidance on which design best supports their objectives. Our experience across Central and Eastern Europe ensures that trials are not only scientifically sound but also operationally viable.
Trial Design Shapes Success
The design of a clinical trial is more than a technical blueprint—it’s the foundation of a successful study. Whether you’re exploring a classic parallel trial design, considering the efficiencies of a crossover trial design, or innovating with platform, basket, or umbrella trial design, the right structure ensures your trial stays on course.
With advancements in clinical trial methodology and increasing opportunities for decentralized clinical trials, sponsors have more tools than ever to design studies that are faster, smarter, and more patient centric.
At Comac Medical, we are ready to support your next trial with the expertise and regional knowledge needed to make your study design a strategic advantage.



