
Planning a clinical trial comes with numerous challenges, requiring a methodical approach for successful execution. However, unforeseen issues like low patient enrollment or budget constraints can surface, making it seem like the clinical trial has failed and is beyond saving. Fortunately, there is a solution: a rescue study.
In this article, we’ll take a deep dive into the essential strategies needed to revive struggling clinical trials, ensuring they get back on track and deliver results. Explore the following key topics to understand how to rescue a trial and avoid future pitfalls. Plus, don’t miss out on our expertise and real-world Case Study that shows how Comac Medical successfully turned a trial around.
What Are Clinical Trial Rescue Studies?
A rescue study is a clinical trial initiated to address unforeseen issues in an ongoing or completed trial. It aims to resolve problems such as data management, patient enrollment issues, and quality control to ensure reliable results. The purpose of a rescue study is essentially to restore the trial’s success, integrity, and reliability, and this can be carried out by either the original CRO providing services or a completely new CRO.
When Do You Need a Clinical Trial Rescue Study Plan?
Determining the need for a clinical trial rescue study relies on assessing the severity of the issues at hand. Early identification of problems like low recruitment or enrollment allows for prompt intervention, potentially saving time and resources by addressing the root causes effectively. Implementing a rescue plan at this stage can prevent minor issues from escalating into major obstacles that disrupt progress and threaten the trial’s success.
A well-prepared rescue plan is crucial for addressing these challenges. It involves reevaluating recruitment goals, amending flawed protocols, and enhancing data management processes. This strategic approach also optimizes resource allocation, addressing shortages that may prevent progress. Identifying and tackling these issues early is key to a successful intervention. Although clinical trial failures present significant challenges, they do not necessarily suggest a trial is unsalvageable. With a clear understanding of the issues and a well-structured rescue strategy, sponsors can regain control of their trial, ensuring it gets back on track and meets its intended objectives. Early intervention and a targeted rescue plan can transform potential setbacks into opportunities for success.

Common Causes of Clinical Trial Failures
Flawed Protocol Design
Understanding the reasons behind clinical trial failures is crucial for determining whether a rescue study is necessary. One of the most significant clinical trial issues is a flawed protocol design. Once a protocol has been agreed upon, it can be difficult to amend, especially when those writing it lack the clinical experience that service providers possess. This often leads to the creation of overly impractical protocols, such as setting high recruitment targets for a study investigating a rare disease. Such targets are often unattainable, leading to failures in recruitment, enrollment, and retention. This results in wasted resources, including expired supplies and team members diverting their attention to other studies. Ultimately, a poorly designed protocol can cause significant setbacks for the entire trial—setbacks that could have been avoided with more meticulous discussions during the planning stage.
Failure to Meet Enrollment Targets
Failure to meet enrollment targets is another common problem in clinical trials, often caused by a variety of factors such as an overestimation of the eligible patient pool and high dropout rates. One key factor contributing to this problem is complex inclusion/exclusion criteria. If these criteria are too difficult to meet, recruitment and enrollment rates will suffer, forcing sponsors to add more sites to meet their targets. This not only increases costs but can also lead to the reduction of other areas, such as specific study tests, due to insufficient funds covering new sites. Consequently, this can affect data integrity and compromise the overall quality of the investigation.
Inadequate Clinical Data Management
Another significant cause in clinical trial failures is inadequate clinical data management, which can compromise both the quality of the trial and the integrity of the data. Clinical Research Organizations (CROs) often use a combination of paper-based and electronic Clinical Data Management Systems (CDMS). Typically, case report forms (CRFs) are initially completed on paper and later transcribed into electronic CRFs (eCRFs) to document protocol details and patient information. Since most trial data is recorded this way, one major issue that can arise is the risk of missing or incorrectly entered data. Ensuring accurate and focused data collection is critical because once the information is uploaded into the eCRF and shared with the sponsor for analysis, any inaccuracies can lead to significant problems. These may include compromised data quality, inefficiencies in the trial process, and potential issues with data security.
Inaccuracies make it hard to determine key outcomes, like whether there was a health improvement, if the hypothesis holds, or if the medication worked. Without reliable data, drawing valid conclusions is nearly impossible, putting the trial at risk. Addressing these challenges early on, through careful protocol design, realistic recruitment targets, and rigorous data management, is essential to ensuring the success of clinical trials and minimizing the risk of failure.

Effective Clinical Trial Rescue Strategies
When a clinical trial encounters issues that threaten its progress, implementing a rescue plan is essential. Whether managed by the current CRO or a new one, we’ll explore key strategies for developing an effective clinical trial rescue study plan to recover and move the trial forward.
Comprehensive Trial Assessment
The assessment is a crucial first step in recovering a clinical trial. This step involves a detailed review of all data and an in-depth analysis of the current protocol. Timeliness is essential, but the assessment must also be thorough to identify and address the root causes of the issues.
Audits play a key role in this process, evaluating site performance, data quality, and patient recruitment. This includes assessing how sites recruit patients, their communication methods, and their management of patients post-enrollment. The audit also examines data collection and transcription methods, including both electronic and paper-based forms, and the handling of samples in the laboratory.
A thorough assessment is vital to prevent further complications. If a rescue plan is implemented without a comprehensive assessment, it may encounter unforeseen issues. Therefore, identifying potential problems early allows for a strategic plan to be developed, minimizing risks and ensuring the trial can proceed effectively.
Strategic Rescue Plan
Effective strategic planning is crucial following a trial assessment to address the clinical trial challenges and ensure its success. This involves crafting a strategic rescue plan designed to identify and rectify issues while ensuring the trial’s success. The plan must be precise, time-efficient, and set realistic goals that the CRO can achieve. Clear communication with the CRO is essential to ensure these objectives are well-understood and actionable.
Optimizing the study protocol is a central focus of the rescue plan strategy. This might involve simplifying inclusion and exclusion criteria to broaden patient eligibility, adjusting dosing regimens to improve efficacy or safety, and modifying endpoints to ensure they remain relevant and achievable given the current challenges. Revitalizing patient recruitment and retention is another critical aspect of the clinical trial rescue services. Strategies to boost recruitment may include targeted outreach efforts, involvement of patient advocacy groups, and expanding eligibility criteria. To enhance retention, providing additional support to patients and offering incentives can significantly improve engagement and reduce drop-out rates.
Improving data management and monitoring is also essential. Implementing improved monitoring systems and leveraging advanced technologies for data tracking can help maintain high data quality and support clinical trial progress. Regular audits and checks ensure that data accuracy is maintained, facilitating effective rescue of study data and addressing issues promptly. The approach to this must be a meticulously developed strategy that addresses the trial’s specific issues, which helps align the study with practical realities and improve its feasibility.
Swift Resource Deployment
Immediate action is required once the rescue plan is completed, this involves strategically placing in-house experts who can reinforce the study’s new infrastructure. These experts are equipped to handle both operational and regulatory challenges that may arise during the trial. By swiftly addressing these issues, the team ensures that the trial’s progress remains on track and that potential setbacks are mitigated effectively.
The focus is on deploying resources in a way that maximizes impact without compromising efficiency. This approach helps maintain the trial’s momentum, ensuring that critical aspects of the study are managed seamlessly. With dedicated support in place, the study can overcome the obstacles more effectively, aligning with overall clinical trial rescue strategies and enhancing the likelihood of successful outcomes. This rapid deployment of resources is essential for navigating complex trial environments and addressing the prior challenges with realistic objectives in place.

Stakeholder Collaboration & Communication
Effective collaboration and communication among sponsors, CROs, investigators, and other stakeholders are crucial for the success of rescue studies. Open and transparent communication fosters a collaborative environment where all parties are aligned and informed. This is especially important whether the rescue study is managed by the original CRO or a new one. A strategic communication plan is often in place, but its success depends on adherence by all involved, including new or shifting staff members who may need time to fully understand the rescue study’s protocol and decision-making processes.
To realign goals and expectations, it is essential to maintain clear and consistent communication. Key aspects include transparent sharing of rescue study data and ensuring that all stakeholders are updated on clinical trial progress and any changes. Regular meetings and updates are effective tools for maintaining alignment and addressing any emerging issues. If communication breakdowns, such as infrequent data sharing, are not addressed, it can lead to misunderstandings and misaligned expectations, ultimately impacting the success of the clinical trial rescue strategies.
Promoting accountability through regular check-ins and fostering an open dialogue will help prevent problems and ensure that the clinical trial rescue services are executed effectively. By ensuring everyone is on the same page, the likelihood of successfully recovering the clinical trial is greatly enhanced.
Regulatory Review & Continuous Improvement
Engaging with regulatory bodies during a rescue study plan requires a thoughtful and strategic approach to ensure compliance and facilitate successful protocol adjustments. The process begins with the preparation of detailed documentation. This documentation should clearly state the necessity of the clinical trial rescue strategies, outline the proposed changes to the protocol, and describe the steps to be taken. It’s essential to address any variations that might arise due to differences in country regulations or transitions to new CROs or study sites.
Maintaining regulatory compliance is crucial throughout the rescue study process. Submitting the prepared documentation to relevant regulatory authorities is a critical step, as it ensures that all necessary approvals are obtained before implementing any changes. This process helps to align the study with regulatory expectations and avoids potential compliance issues.
Clinical trial progress should be closely monitored through a robust monitoring system to oversee that the revised protocol is being followed accurately. Any issues or deviations need to be promptly addressed to maintain the study’s integrity and regulatory adherence. This ongoing oversight helps to recover clinical trial performance and maintain the study’s integrity
By focusing on continuous review and improvement, you can navigate the regulatory landscape effectively. This approach not only helps in managing compliance but also enhances the likelihood of a successful clinical trial rescue. Continuous communication and detailed review contribute to ongoing improvements, ensuring that the rescue study data is accurate and that the trial is completed successfully and meets its objectives.

Selecting the Right Rescue CRO for Your Study
Selecting the right CRO to provide rescue study services is crucial. Sponsors must carefully evaluate several key factors to ensure they choose a CRO that can effectively address and resolve the issues at hand.
Experience in Trial Rescue
Firstly the CRO should have a proven track record in rescuing clinical trials. This experience is invaluable as it indicates their ability to develop a comprehensive plan for transition and recovery. Assessing their history of handling similar situations and reviewing case studies or testimonials from previous clients will provide insight into their success rates.
Relevant Resources
The chosen CRO must possess the specific resources and expertise relevant to the study’s current challenges. Whether the issue lies in data management, patient recruitment or trial design, the CRO must have the right tools and personnel to tackle these areas effectively. It is essential to verify that their capabilities match the unique needs of your study. Additionally, consider whether the CRO can offer improved services compared to the original CRO. This includes enhanced communication practices, superior data management systems, and more effective patient recruitment and enrollment strategies. The goal is not just to salvage the trial but to enhance its overall execution and outcome.
Alignment with Specific Needs
Lastly, while a CRO might excel in rescue services, their expertise may vary across different aspects of a study. Ensure that their strategies align with your specific needs, for example if data management is a primary concern, the CRO should have an effective data system in place to address this issue.
Comac Medical Rescue Study Expertise
Comac Medical offers extensive expertise in providing rescue study services, delivering flexibility as either a ‘companion CRO’ or assuming full responsibility for study management. Our comprehensive approach to trial transition involves a detailed review and assessment of all study components to identify and address key issues hindering clinical trial success. We develop targeted rescue plans that tackle current challenges, expedite schedules, and ensure a complete and effective solution.
Our rescue study solutions include integrating and aligning clinical trial services, systems, and logistics while maintaining clear communication with the sponsor. We also manage transition costs, milestones, and resources to ensure a smooth and efficient budget analysis process.
Multiple Myeloma Case Study – Rescue Study by Comac Medical
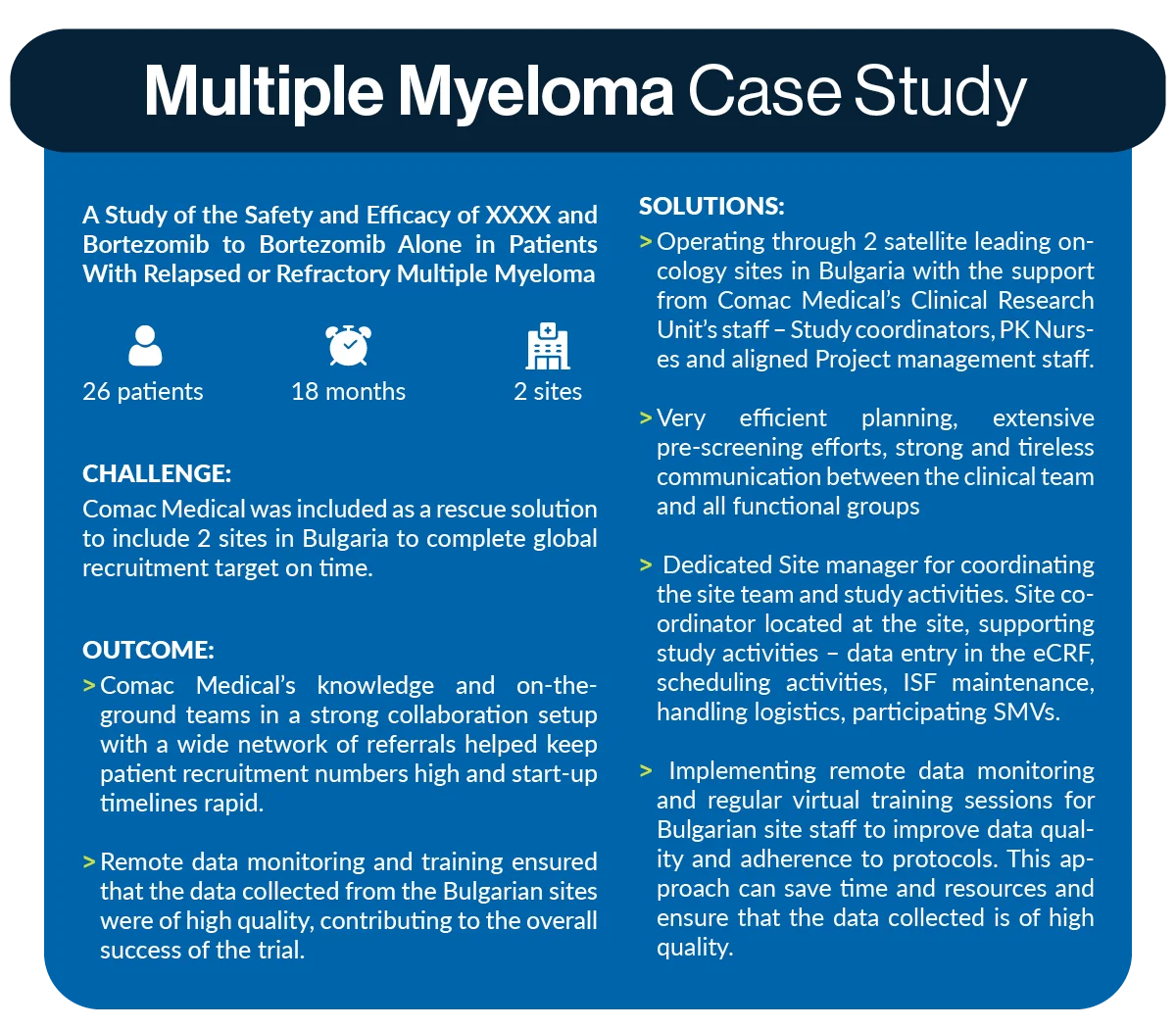
Study Summary:
A study of the safety and efficacy of XXXX and Bortezomib to Bortezomib alone in patients with relapsed or refractory Multiple Myeloma – 26 patients were recruited in 18 months, 2 sites.
Challenges:
Comac Medical was included as a rescue solution to include 2 sites in Bulgaria to complete the global recruitment target on time.
Solutions:
- Operating through 2 satellite leading oncology sites in Bulgaria with the support from Comac Medical’s Clinical Research Unit’s staff – Study coordinators, PK Nurses and aligned Project management staff
- Very efficient planning, extensive pre-screening efforts, strong and tireless communication between the clinical teams and all functional groups
- Dedicated Site manager for coordinating the site team and study activities. Site coordinator located at the site, supporting study activities – data entry in the eCRF, scheduling activities, ISF maintenance, handling logistics, participating SMVs
- Implementing remote data monitoring and regular virtual training sessions for Bulgarian site staff to improve data quality and adherence to protocols. This approach can save time and resources and ensure that the data collected is of high quality.
Outcome:
Comac Medical’s knowledge and on-the-ground teams in a strong collaboration setup with a wide network of referrals helped keep patient recruitment numbers high and start-up timelines rapid. Remote data monitoring and training ensured that the data collected from the Bulgarian sites were of high quality, contributing to the overall success of the trial.
At Comac Medical, we recognize the inherent stress and complexity involved when a sponsor or CRO is struggling to complete a trial. Our rescue study expertise is designed to alleviate this pressure and deliver optimal results, turning challenging situations into successful outcomes.
If you’re experiencing difficulties with your clinical trial and need support, contact us for a consultation with our expert team to explore the solutions you need.



