For small to mid-sized biotech companies, navigating the complexities of clinical trials can be challenging. With limited resources, tight timelines, and the need for precise execution, ensuring the success of a clinical trial requires careful planning. One of the most critical steps in this process is conducting a thorough clinical trial feasibility assessment before initiating the trial itself. This crucial stage helps biotech companies evaluate the practical, regulatory, and operational aspects of their clinical trial, minimizing risks and setting the foundation for success.
In this article, we’ll explore why feasibility assessments are essential, the role of Contract Research Organizations (CROs) and we’ll provide some practical tips:
1. Understanding Clinical Trial Feasibility Assessment
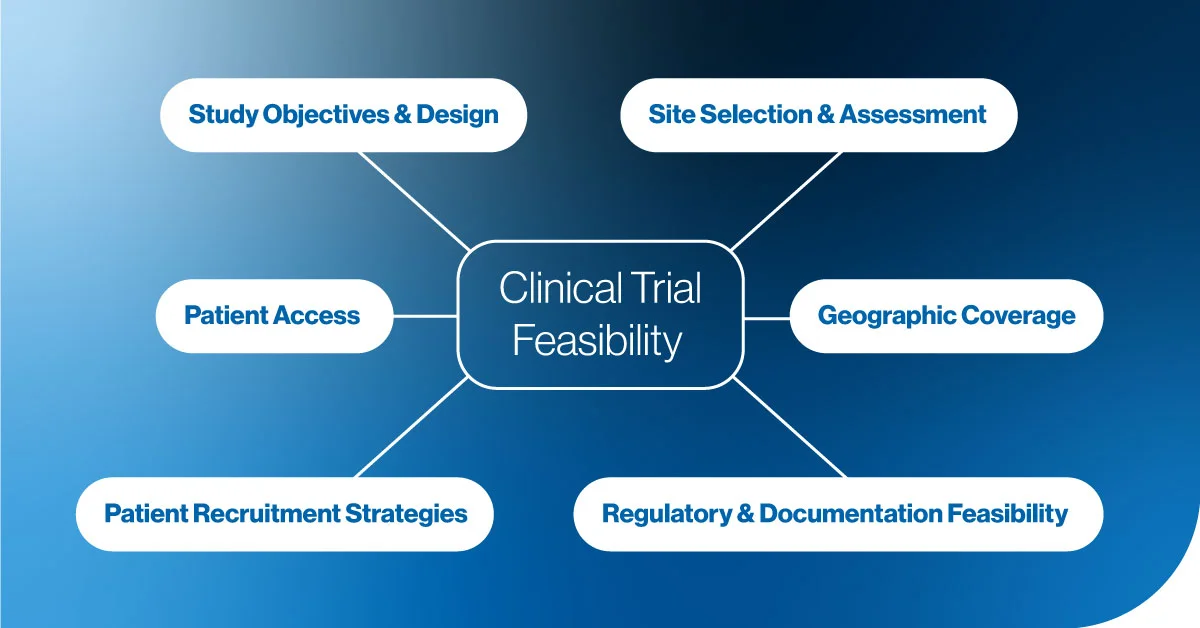
At its core, clinical trial feasibility assesses the practical viability of a trial by examining key factors such as site selection, patient recruitment, and regulatory requirements. It provides a roadmap for avoiding common pitfalls, hence, identifying potential challenges early in the process. For biotech companies, particularly those with innovative therapies, understanding feasibility is critical to making informed decisions before starting a clinical trial.
Clinical Trial Site Feasibility
Choosing the right clinical sites is one of the first and most important steps in feasibility. The right site not only provides access to the target patient population but also facilitates the resources and infrastructure to ensure trial efficiency. At Comac Medical, we thoroughly assess each site’s suitability based on experience, location, and investigator expertise.
Patient Access and Recruitment
Patient recruitment is often the most challenging aspect of any clinical trial, especially in biotech, where the target patient populations may be smaller or more specific. A strong feasibility assessment evaluates whether the trial can recruit the required number of patients in the planned timeline and proposes tailored strategies to address recruitment challenges.
Regulatory and Documentation Feasibility
Navigating regulatory approvals and adhering to local requirements can cause significant delays if not planned carefully. A proper feasibility assessment ensures that all regulatory documentation is in place, local guidelines are respected, and approval processes are factored into the project timeline.
Clinical Trial Feasibility Assessment in Early-Phase Trials
Feasibility is particularly important in early-phase trials (Phase I), where the risks and complexities are higher. A detailed feasibility assessment helps biotech companies anticipate and mitigate these risks, ensuring that early-phase trials are executed efficiently and that potential issues are resolved before they escalate.
2. Clinical Trial Feasibility Challenges
For small and mid-sized biotech companies, conducting a comprehensive feasibility assessment is not just an optional step—it is a critical safeguard against potential trial setbacks. The process identifies risks, such as regulatory hurdles, patient recruitment challenges, and operational inefficiencies, before they escalate into costly delays or trial failures. By pinpointing these issues early, feasibility assessments enable biotech companies to better allocate resources, adjust trial protocols, and streamline execution, setting the stage for smoother operations and a higher likelihood of trial success.
Limited Financial and Operational Resources
Small biotechs frequently lack the vast budgets that larger companies can allocate to clinical trials. This restricts their ability to pivot quickly when faced with unforeseen complications, such as site recruitment delays or patient retention issues. A poorly executed feasibility assessment can exacerbate these financial constraints by underestimating trial costs or failing to identify resource bottlenecks early.
Regulatory Complexity
Regulatory compliance is a significant challenge for biotech companies, particularly those entering multiple regions with varying requirements. Navigating the maze of local and international regulations can be overwhelming, especially for biotechs without an established regulatory team. A detailed feasibility assessment helps anticipate these regulatory demands, ensuring that all necessary documentation and approvals are secured in a timely manner, avoiding lengthy delays.
Patient Recruitment and Retention
One of the most underestimated risks in clinical trials is patient recruitment and retention. For small biotechs, which often target niche therapeutic areas or rare diseases, finding and enrolling the right patients can be extremely challenging. A solid feasibility assessment can help evaluate whether the patient population is accessible and if there are enough resources to support recruitment efforts. Without this insight, trials risk stalling due to a lack of participants, leading to extended timelines and additional costs.
Unfamiliarity with Clinical Trials
Many small biotech companies are led by scientific teams with deep expertise in drug development but limited experience in the operational aspects of running clinical trials. This lack of familiarity can lead to missteps, such as underestimating timelines, failing to secure reliable clinical sites, or overlooking the importance of patient engagement strategies. An experienced CRO conducting a feasibility assessment can fill these knowledge gaps, offering guidance that reduces the likelihood of operational mishaps.
These challenges highlight the necessity of partnering with a CRO that understands the intricacies of clinical trial feasibility and can provide an adequate clinical trial feasibility assessment. With expert guidance, small and mid-sized biotech companies can not only navigate the complexities of clinical trial planning but also gain confidence that their trials are designed for success from the outset. Comprehensive feasibility assessments help minimize risks and provide the strategic foresight needed to ensure that trials are executed efficiently and cost-effectively.
To help biotech companies get started, we’re offering a Free Feasibility Session, followed by a Free-of-Charge Feasibility Report. This offer allows you to receive a detailed analysis of your trial’s potential before making any long-term commitments.

3. Selecting the Right CRO for A Clinical Trial Feasibility Assessment
Choosing the right Contract Research Organization (CRO) for your clinical trial feasibility assessment is a critical decision, especially for biotech companies. Biotech trials often involve complex therapies and niche patient populations, requiring a CRO that offers both specialized expertise and personalized service. While large CROs may provide broad resources, smaller and mid-sized CROs can offer more flexibility, direct attention, and tailored support that better aligns with the needs of biotech companies.
Why Mid-Sized CROs Are the Best Choice for Biotech Clinical Trials & Feasibility Assessment
✅ Proven Expertise in Biotech Trials
Mid-sized CROs often have highly specialized teams with focused expertise in niche therapeutic areas. This is particularly valuable for biotech companies conducting trials with complex treatments or targeting small, specific patient populations. With concentrated experience in biotech, these CROs understand the unique challenges of biotech clinical trials, from regulatory challenges to patient recruitment.
✅ Personalized Attention for Better Outcomes
Unlike larger CROs, mid-sized CROs typically offer more personalized service, with direct access to senior decision-makers and dedicated teams. This can result in faster decision-making and clearer, more effective communication between the CRO and the biotech company. Personalized attention ensures that the CRO team is deeply involved in the trial, leading to quicker issue resolution and more efficient trial management.
✅ Direct Connections with Clinical Sites
Mid-sized CROs often have direct and longstanding relationships with clinical sites. This can streamline site selection and ensure that any issues encountered during the trial can be addressed quickly, minimizing the risk of delays. Direct site connections also enable more responsive patient recruitment and faster start-up times, crucial for keeping the trial on track and within budget.
✅ Minimizing Vendor Complexity
Many biotech companies find that working with fewer vendors during a clinical trial can significantly enhance efficiency. Mid-sized CROs offer comprehensive services, reducing the need for multiple vendors and ensuring seamless communication between all stakeholders. This streamlined approach decreases the risk of miscommunication and improves trial coordination.
✅ Biotech Clinical Trial Feasibility Assessment
When it comes to feasibility assessments, larger CROs often struggle with the agility and focus needed for biotech-specific trials. The multiple layers of management and extensive portfolio of clients can lead to generalized assessments that fail to account for the unique challenges of small biotech trials. This can result in inaccurate patient recruitment projections, site selection delays, and misalignment with regulatory requirements. In contrast, mid-sized CROs are able to deliver more tailored feasibility assessments, offering biotech companies in-depth analysis, faster response times, and a focused approach that addresses the specific complexities of their clinical trials from the very start.
To help biotech companies get started, we’re offering a Free Feasibility Session, followed by a Free-of-Charge Feasibility Report. This offer allows you to receive a detailed analysis of your trial’s potential before making any long-term commitments.

4. Top 10 Important Questions to Ask Your CRO During a Feasibility Meeting
When biotech companies engage with a CRO for a feasibility assessment, asking the right questions can make all the difference in ensuring the trial’s success. A well-conducted feasibility meeting not only sets the stage for a successful collaboration but also helps identify potential risks and ensures that the clinical trial is designed and managed with precision. Here are ten essential questions to ask during your feasibility meeting to ensure you’re making the most informed decisions:
- ➡️ What is Your Experience with Similar Trials? Ask the CRO about their specific experience in conducting trials similar to yours, particularly in your therapeutic area. Understanding their past performance can provide insight into their expertise and ability to address the unique challenges of your study.
- ➡️ How Do You Approach Site Selection? Site selection is critical for a trial’s success. Inquire about their process for choosing clinical sites and how they ensure that the selected sites have access to the appropriate patient populations and necessary infrastructure.
- ➡️ What Are Your Strategies for Patient Recruitment and Retention? Effective patient recruitment can be a major challenge in clinical trials. Ask the CRO about their recruitment strategies, including how they engage potential patients, minimize dropouts, and ensure enrollment targets are met on time.
- ➡️ How Do You Ensure Compliance with Regulatory Requirements? Each country or region has unique regulatory demands. Ask the CRO about their strategies for navigating complex regulatory landscapes and ensuring that the trial adheres to all relevant guidelines to avoid costly delays.
- ➡️ Can You Provide Examples of Past Feasibility Success? Request case studies or examples where their feasibility assessments have led to successful trials. This can give you a sense of how the CRO tackles challenges and how proactive they are in addressing potential issues early.
- ➡️ What Are Your Contingency Plans for Potential Delays or Challenges? Clinical trials rarely go perfectly according to plan. Ask the CRO how they address unforeseen issues, such as recruitment slowdowns, regulatory hurdles, or operational inefficiencies, and whether they have contingency plans in place.
- ➡️ How Will You Ensure Clear Communication Throughout the Trial? Effective communication between the biotech company and the CRO is essential for a smooth trial. Ask how they plan to maintain transparent, frequent, and effective communication throughout the process, including regular updates and problem-solving protocols.
- ➡️ How Do You Assess the Feasibility of Reaching Enrollment Targets? Understanding the CRO’s approach to enrollment projections and how they assess the patient population at each site is crucial. Ask how they evaluate the feasibility of meeting recruitment goals and what measures they take if enrollment is slower than expected.
- ➡️ What Technologies Do You Use to Monitor and Manage the Trial? Technology plays a key role in ensuring clinical trial efficiency and transparency. Ask about the systems they use for trial management, including data collection, monitoring, and reporting, to ensure you have real-time access to critical information.
- ➡️ What Level of Involvement Will Senior Staff Have in My Trial? For small and mid-sized biotech companies, direct access to senior CRO staff can be a major advantage. Ask how involved senior staff will be in your trial, including decision-making and oversight, to ensure the highest level of expertise is guiding the study.
5. Actionable Tips for Maximizing Feasibility in Biotech Trials
In addition to asking the right questions, here are a few actionable tips for biotech companies to ensure their feasibility assessments are thorough and lead to success:
- ⭐ Prioritize Early Collaboration: Engage your CRO as early as possible to ensure that the feasibility assessment takes all critical factors into account. Early involvement allows more time for detailed planning and problem-solving before trial execution begins.
- ⭐ Involve Key Stakeholders: Ensure that both internal teams and external experts (such as regulatory consultants or patient advocacy groups) are part of the feasibility discussions. Their insights can help you anticipate challenges that may not be immediately visible.
- ⭐ Tailor Your Feasibility Assessment: Customize the feasibility process to reflect the specific needs of your biotech company, the trial design, and the therapeutic area. One-size-fits-all assessments may overlook critical nuances, leading to future issues.
- ⭐ Request Regular Updates: Establish a protocol for regular feasibility updates, particularly during the early stages of the trial. Regular communication helps ensure that any issues are detected and resolved before they become significant roadblocks.
- ⭐ Utilize Predictive Tools: Consider leveraging tools like feasibility calculators or simulation models that predict trial timelines, costs, and enrollment likelihoods. These tools can provide valuable data to help guide decisions during the feasibility process.
6. Comac Medical’s Free Personalized Feasibility Session to Support Your Next Trial
No two clinical trials are the same, and your feasibility assessment should be tailored to your specific needs. This includes considering the therapeutic focus, trial design, patient population, and geographic scope of your study. At Comac Medical, we offer a Free Personalized Feasibility Session to help biotech companies navigate this critical stage with confidence.
Why Choose Comac Medical for Your Feasibility Assessment?
Our tailored approach evaluates every aspect of your study, from trial objectives and site selection to patient recruitment and regulatory considerations. As part of this exclusive offer, you’ll receive a Free-of-Charge Feasibility Report, complete with actionable insights to enhance your trial’s performance.
What You’ll Gain:
- Expert Guidance: Work directly with seasoned industry professionals, including:
- – Igor Bogdanoski: Feasibility Manager with over 10 years of expertise in site evaluation and risk mitigation.
- – Hrabrina Hristova: Regulatory leader with over 15 years of experience ensuring compliance.
- – Dr. Monica Stanescu: Medical Monitor Team Lead specializing in clinical oversight and trial design.
- Comprehensive Support: Benefit from expert-driven recommendations covering study design, geographic analysis, and patient strategies.
- Optimized Outcomes: Maximize trial efficiency, mitigate risks, and ensure long-term success.
Ready to Get Started?
Schedule your Complimentary Feasibility Session today and take advantage of this opportunity to receive expert insights and a detailed Feasibility Report tailored to your trial’s needs. Let Comac Medical be your trusted partner in achieving success.