With the ongoing evolution of global healthcare, identifying strategic locations for clinical trials has become a vital component in accelerating drug development. As the demand for newer, more effective drugs continues to grow, so does the necessity of cost-efficient, high-quality locations for conducting clinical trials. In recent years, pharma and biotech companies have increasingly looked beyond traditional markets to regions offering economic advantages and access to diverse patient populations.
Among these non-traditional regions, Central and Eastern Europe (CEE) has emerged as a highly attractive destination for clinical trials, with a noticeable rise in activity over the past decade. Thanks to their robust healthcare infrastructure, skilled investigators, and streamlined regulatory processes, countries such as Poland, Hungary, the Czech Republic, Slovakia, Romania, and Bulgaria are becoming leaders in clinical trial conduct. While still underutilized, the increase of clinical trial trials in Central and Eastern Europe (CEE) highlights the untapped potential of this region, offering high-quality clinical data alongside significant cost savings.
This article will explore the key insights, opportunities, and benefits of conducting clinical trials in Central and Eastern Europe, covering different aspects:

Understanding the Clinical Trials Landscape in Central and Eastern Europe (CEE)
Contract Research Organizations (CROs) are integral to the clinical trial process, offering a comprehensive suite of services, including trial design planning, site selection, patient recruitment and retention, clinical monitoring, regulatory affairs, and data and project management. These specialized services are essential for managing complex clinical trials and supporting the product development lifecycle from concept to regulatory approval.
As clinical research grows more sophisticated, sponsors increasingly rely on CROs to navigate innovative trial designs, such as basket trials, and meet rigorous regulatory standards. The CRO industry has thus become a cornerstone of modern drug development, offering faster, cost-effective solutions without compromising quality.
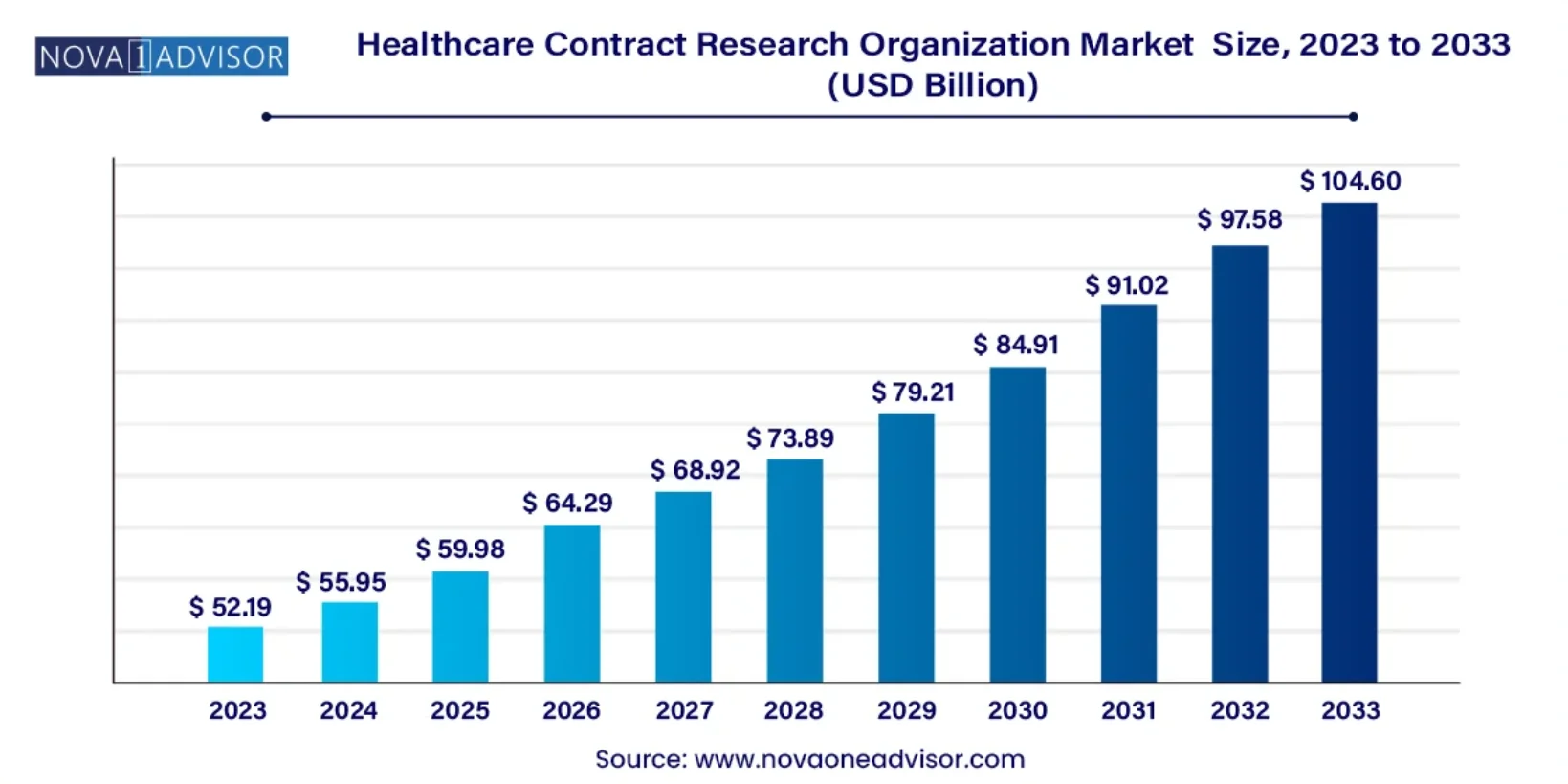
In 2023, the global CRO market was valued at USD 52.19 billion and is projected to grow at a compound annual growth rate (CAGR) of 7.2%, reaching approximately USD 79.21 billion by 2029 and USD 104.60 billion by 2033. This remarkable growth reflects the growing reliance on CROs to manage emerging trends like precision medicine and patient-centric trial designs, which demand advanced trial management strategies and adherence to strict regulatory standards.
Regions that combine expertise with cost-effective solutions are increasingly becoming focal points for clinical research. Amid this dynamic landscape, CEE has emerged as a strategic hub for clinical trials. With its high-quality infrastructure, diverse and accessible patient populations, and cost advantages, the region offers an optimal environment for running efficient and effective clinical studies.
The rise of European CRO services, such as Comac Medical, has further highlighted the region’s capabilities. These organizations have demonstrated the ability to recover delayed or underperforming trials with confidence, delivering reliable results within tight timelines, making them particularly well-suited for clinical trial rescue studies.
In the following sections of this article, we will explore the factors driving the CEE region’s emergence as a preferred destination for clinical trials, highlight its key advantages, and debunk common misconceptions about conducting research in this area.
Benefits of Conducting Clinical Trials In Central and Eastern Europe (CEE)
1. Access to a Diverse and Treatment-Naïve Population
Central and Eastern Europe (CEE) has emerged as a preferred destination for clinical research, offering distinct advantages that set it apart from other regions. From patient diversity to untapped populations, CEE provides a fertile ground for conducting efficient and impactful clinical trials.
Large and Diverse Patient Pool
A major advantage of conducting clinical trials in Central and Eastern Europe is the region’s access to a large and diverse patient pool. With a combined population of approximately 250 million, CEE offers an unique opportunity to recruit participants across varied demographics, including different age groups, socioeconomic backgrounds, and medical histories. This diversity enhances the applicability of clinical trial results while promoting the inclusion of underrepresented groups in global research efforts.
The increased prevalence of chronic diseases such as diabetes, cardiovascular disease, and cancer reinforces patient recruiting efforts ensuring that trials targeting these specific therapeutic areas can find eligible participants quickly and efficiently. In contrast to more saturated locations, CEE’s clinical research landscape is not impeded by overwhelming patient competition, resulting in speedier enrollment with fewer delays.
Treatment-Naïve Participants
Moreover, CEE offers access to a niche advantage: treatment-naïve populations. Compared to Western Europe or the United States, many patients in CEE have not been extensively exposed to prior clinical trials or advanced therapies. Readily available treatment-naïve populations means that there is a decreased possibility of confounding variables developing, such as previous treatment effects which can complicate data interpretation. This results in cleaner, more reliable, free-from-bias data which is highly beneficial for sponsors as this can accelerate regulatory reviews and further decision-making processes.
Leveraging the patient pool in CEE offers pharmaceutical and biotechnology companies a strategic ‘upper hand’ that is hard to find elsewhere if they intend to get credible results while maintaining efficient operations.
2. Faster Patient Recruitment and Retention
Recruitment Efficiency
Efficient patient recruitment remains one of the most critical challenges in clinical research, and CEE excels in overcoming this hurdle. Unlike more saturated markets in Western Europe or the United States, where sponsors often compete for the same patient pools, the CEE region benefits from significantly fewer competing trials, leading to efficient and rapid enrolment.
The recruitment procedure is enhanced by dedicated, proactive, highly competent investigators. Further, the success of clinical research is vital to many scientists in the area, and they frequently work closely with trial sponsors to accomplish their enrollment targets.
High Retention Rates
High patient retention rates in CEE add another layer of efficiency to clinical trial management. Cultural and socioeconomic factors play an important role here, as patients in central and eastern Europe tend to place a high value on healthcare access and the benefits associated with participating in cutting-edge clinical trials. This leads to strong adherence to trial protocols, reducing the risks of dropout and ensuring the integrity of collected data.
Investigators sustain meaningful working connections with their patients, cultivating trust and a sense of commitment that results in consistent trial participation. Together, these factors create an ecosystem where patient recruitment and retention operate seamlessly, minimizing delays and maximizing the reliability of trial outcomes.

3. Cost-Effective Trial Operations: Maximizing Efficiency Without Compromising Quality
Cost efficiency is another defining strength of conducting clinical trials in collaboration with European clinical research organizations. Compared to Western Europe and the United States, Eastern and Central European CRO services, such as Comac Medical’s CRO services, offer substantial cost advantages. Тhis is achieved through lower investigator fees, reduced site expenses, and more economical patient reimbursements. Therefore, the CEE is a desirable option for sponsors looking to maximize budgets without foregoing quality.
Despite these lower costs, the CEE region boasts top-tier research facilities equipped with state-of-the-art technology and highly qualified professionals, including experienced investigators and site staff. This ensures that trials conducted in CEE meet the highest global standards while remaining economically accessible.
The cost-effectiveness of CEE trials also extends to regulatory processes, which are streamlined and well-aligned with EU and global standards. This not only reduces administrative burdens but also contributes to faster trial startup times, saving both time and resources. For sponsors, this unique combination of affordability and quality makes the region an ideal choice for conducting robust, reliable clinical trials while optimizing financial and operational efficiency.
4. Expertise and Infrastructure Driving Clinical Trial Excellence
Experienced Clinical Trial Professionals in CEE
The CEE region boasts a wealth of experience, investigators and study coordinators who bring extensive clinical trial expertise to the table and contrary to the popular belief, almost all the investigations, healthcare professionals and clinical trial management team have very strong English-speaking skills.
Many of these professionals have perfected their skills by collaborating on international studies and are adept at meeting rigorous global legislations. Alongside this, Central and Eastern Europe offer a plethora of prestigious medical institutions which guarantee an abundance of highly-qualified, passionate professionals possessing the knowledge and expertise needed to provide valuable additions to ambitious clinical research ventures.
Cutting-Edge Facilities Driving CEE Clinical Trial Excellence
The state-of-the-art infrastructure, such as Comac Medical’s Early Phase Clinical Research Unit, facilitates fast-paced and efficient clinical trial conduct and perfectly complements the exceptionally competent workforce. Numerous research sites in CEE are equipped with advanced diagnostic tools, laboratories, and compliant data management systems, ensuring precision and reliability in trial execution. Integration of cutting-edge technologies enables sites to handle complex protocols and large datasets with ease, consistently surpassing international standards and providing sponsors with the assurance of high-quality data collection and reporting.
Additionally, the infrastructure is supported by efficient healthcare systems that facilitate smooth coordination between trial sites and regulatory authorities, streamlining the overall trial process. This distinctive combination of expertise and infrastructure presents a framework that is optimal for clinical research success.
5. Regulatory Alignment with the EU and Global Standards
Harmonized Regulatory Framework
The CEE region encompasses a diverse mix of countries, including EU members like Bulgaria and non-EU countries such as North Macedonia. However, this diversity does not pose a challenge, as all countries in the region are well-acquainted with the legislation and regulations of their neighbors. This familiarity ensures smooth and efficient operations across borders, fostering seamless collaboration and progress in clinical trial activities.
For countries within the European Union, adherence to the EU Clinical Trials Regulation ensures a harmonized framework for trial approvals. This alignment with EU-wide standards guarantees consistency and transparency, streamlining processes from the initial submission to the execution of trials. Sponsors can rely on a regulatory system that prioritizes patient safety and data integrity while facilitating efficient trial progression. In non-EU countries across the CEE region, local regulatory frameworks align closely with global standards such as Good Clinical Practice (GCP) and the International Council for Harmonisation (ICH) guidelines. This alignment ensures that trials conducted in these countries meet the rigorous requirements demanded by global regulatory authorities. Sponsors benefit from a seamless integration of compliance measures, making it easier to consolidate data and maintain uniform quality across multinational studies.
Efficient Approval Processes
A standout feature of the regulatory landscape in CEE is the streamlined approval process. Ethics committees and regulatory authorities in the region often operate on shorter review timelines compared to some Western markets. This expedited process enables faster trial initiation, reducing delays and offering sponsors the opportunity to meet project milestones more effectively.
The combination of harmonized regulations, adherence to global standards, and efficient approval timelines creates an environment that fosters the smooth execution of clinical trials. For sponsors navigating the complex and time-sensitive nature of clinical research, these factors provide an invaluable edge, ensuring that studies are conducted efficiently without compromising on quality or compliance.
6. Strategic Location and Connectivity
Proximity to EU and US
CEE is a prime, strategic location offering distinctive advantages for clinical research, particularly for sponsors from the EU and the US. Situated at the intersection of major global markets, the region serves as a bridge for international collaborations. Its central location allows for optimal coordination between sponsors, investigators, and trial sites, enhancing the overall efficiency of clinical trial operations.
Modern transportation networks within CEE ensure seamless access to trial sites, simplifying monitoring visits and fostering closer oversight. The region’s major cities are well-connected by road, rail, and air, thereby easing communication and reducing logistical difficulties. This accessibility minimizes travel hassles while enabling sponsors to remain closely connected with trial progress.
Local Knowledge with Global Reach
Moreover, the CEE region combines local expertise with a global perspective. Many CROs in the area possess an in-depth understanding of the local healthcare and regulatory landscape, ensuring that trials are tailored to specific regional contexts. At the same time, their experience with international standards and cross-border collaborations ensures that global sponsors receive the level of quality and compliance they expect. This dual focus on local knowledge and global reach makes the region particularly appealing for sponsors looking to navigate the complexities of multinational clinical trials.
The region’s location, infrastructure, and specialized support systems perfectly balances accessibility with excellence, offering a productive environment for conducting trials across diverse markets.

Common Misconceptions About Conducting Clinical Trials in Central and Eastern Europe (CEE)
Lack of Regulatory Compliance
❓ Myth: CEE countries have inconsistent or outdated regulatory frameworks.
✅ Reality: Many CEE countries adhere to EU regulations and global standards like GCP and ICH guidelines, ensuring high compliance.
Inferior Infrastructure
❓ Myth: Clinical trial sites in the region lack modern facilities or advanced technology.
✅ Reality: CEE boasts high-quality infrastructure, including state-of-the-art diagnostic equipment and well-equipped laboratories.
Limited Patient Diversity
❓ Myth: The region does not offer a diverse or representative patient population.
✅ Reality: With over 250 million people, CEE provides access to varied demographics, including treatment-naïve populations.
Slower Recruitment Timelines
❓ Myth: Recruitment in CEE is slower due to less awareness about clinical trials.
✅ Reality: The region is known for faster patient recruitment rates, aided by motivated investigators and a less saturated trial landscape.
Language and Communication Barriers
❓ Myth: Language differences hinder efficient communication and trial operations.
✅ Reality: Most investigators and CRO staff in CEE are fluent in English and experienced in managing international studies.
Poor Data Quality
❓ Myth: The quality of clinical data generated in the CEE region is inferior compared to Western Europe or the US.
✅ Reality: Clinical trial sites in CEE consistently meet or exceed international standards, offering data of equal or even higher quality. This is supported by the region’s advanced infrastructure, experienced investigators, and adherence to global guidelines like GCP and ICH.